Abstract
Introduction: Recent data suggest that MDS evolves by accumulating mutations. Early mutations may involve genes that require additional mutations prior to clinical manifestation as MDS. We explored if mutant allele burden and the relative mutation of one gene to another gene could provide information on the interclonal and intraclonal progression of MDS using next generation sequencing (NGS) in patients with early MDS.
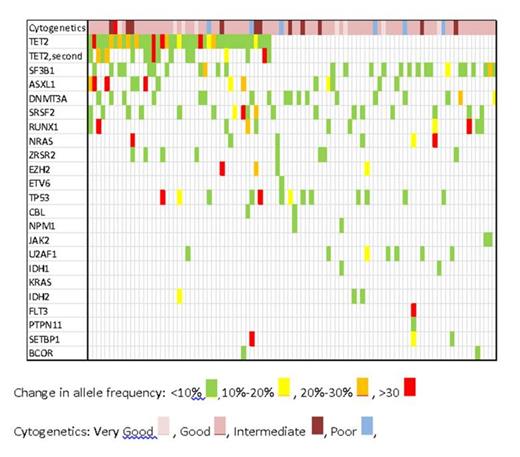
Methods: NGS data was generated from 96 patients diagnosed with MDS with marrow blast count <5% using a targeted sequencing covering mutations in the following genes: TET2, SF3B1, ASXL1, DNMT3A, SRSF2, RUNX1, NRAS, ZRSR2, EZH2, ETV6, TP53, CBL, NPM1, JAK2, U2AF1, IDH1, KRAS, IDH2, FLT3, PTPN11, SETBP1, and BCOR. The average depth of sequencing was 10,000X. Differences in mutant allele frequency between two genes in the same sample were considered significant if they were >10%. A difference of 10% to 20% was considered mild, 20%-30% moderate, and >30% severe. A heat map reflecting these differences in mutant allele frequency was generated.
Results: In this group of early MDS patients, 63 patients (66%) had more than one gene mutated and 38 (40%) had a significant (>10%) difference in allele frequency. The median number of genes mutated was 2 (range 1 to 5). Difference in mutant allele frequency was severe in 15 patients (16%), intermediate in 15 patients (16%), and mild in 13 patients (14%). TET2 was the most commonly mutated gene (43 patients, 45%) and was rarely the sole mutation with most cases exhibiting a mutation in a second gene (39 patients, 91%). The mutant allele burden was highest in TET2 in 26 of these 39 patients (67%), reflecting early event in the tumorigenic process. Of the 13 cases with TET2 mutation and allele burden less than the companion gene, 6 had a mutation in SF3B1, 3 had significant cytogenetic abnormalities (monosomy 5, del(7q), and trisomy 8), 2 had a mutation in SRSF2, 1 had a mutation in ZRSR2 and 1 had a mutation in ASXL1, which suggests that these abnormalities might be the initiating event. A second TET mutation (biallelic mutation) was detected in 16 of the 39 patients. SF3B1 was the most common gene having a solitary mutation (10% of all patients), although mutation in SF3B1 was detected in 27 patients (26% of all patients). All solitary SF3B1 mutations were associated with normal karyotypes, except for one patient with del(11q). JAK2 was mutated with SF3B1 in two cases diagnosed as RARS-T (refractory anemia with ring sideroblasts and thrombocytosis). In one case, the JAK2 and SF3B1 mutation allele frequencies were similar, but in the other, the JAK2 mutant allele frequency was 23% higher, suggesting that a myeloproliferative neoplasm was the initiating process. ASXL1 was mutated in 14 cases, 13 of which had additional mutations. DNMT3A gene was mutated in 18 cases, 5 of which were solitary; two of these five showed cytogenetic abnormalities. TP53 was mutated in 13 cases, but except for one case, all had either mutation in another gene or a cytogenetic abnormality.
Conclusion: These data suggest that in patients with clinically confirmed early MDS, TET2 mutations are most likely the initiating oncogenic event, but mutations in other genes or cytogenetic abnormalities most likely lead to clinically confirmed MDS. In contrast, patients with SF3B1 mutation can have clinical disease without additional mutations. Our data suggest that SRSF2, ZRSR2, and ASXL1 may initiate mutagenesis in patients with MDS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal