Abstract
Background
DHX9(Asp-Glu-Ala-His box helicase 9) is considered to play a key role in many biological processes and cancer development. However, the effect of DHX9 mutation and the main function is not fully understood in myelodysplastic syndromes (MDS).
Objective
The objective of this study is to investigate the frequency of DHX9 mutations and their relationship with clinical characteristics in MDS.
Methods
Next generation sequencing was used to determined the DHX9 mutations and other recurrent genes mutations in 320 MDS patients. The effect of DHX9 mutations or combination with other genes mutations on patients' survival was analyzed. The association of DHX9 mutations with clinical characteristics of MDS was also studied. In addition, the effect of DHX9 on tumor biological features was evaluated in vitro.
Results
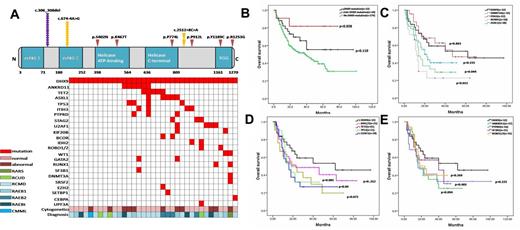
1. DHX9 mutations were detected in 10.94% of MDS patients(35/320). 42.9% of these mutation were del(c.306_308), 31.4% were splicing mutations (c.674-4A>G; c.2512+8C>A) and the rest (25.7%) were missense mutation. In these patients with DHX9 mutations, 24(68.6%) had concomitant occurrence with other mutations such as ANKRD11, TET2, ASXL1, TP53 and U2AF1 (Fig.1A).
2. The patients with DHX9 mutations especially sole mutations had significantly superior survivalcompared with those without mutations (Fig.2B). The patients with DHX9 mutations also had superior survivalcompared with those with genes mutations with poor prognosis, such as DNMT3A/RUNX1/ASXL1, IDH1/TP53/U2AF1, PTPRD/ANKRD11, even those mutations with good prognosis such as TET2 and SF3B1 (Fig.2C, 2D and 2E).
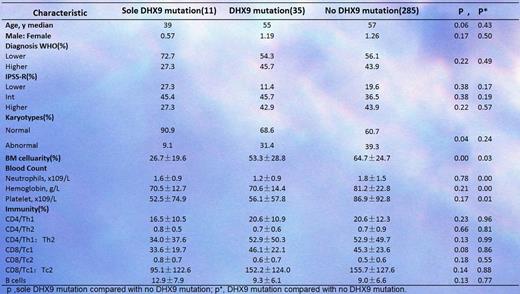
3. We also analyzed the difference in clinical features between the patients with and without DHX9 mutations (Table 1). Obviously, the patients with DHX9 mutations exhibited more severe BM failure and reduced Tc1/Tc2 polarization.
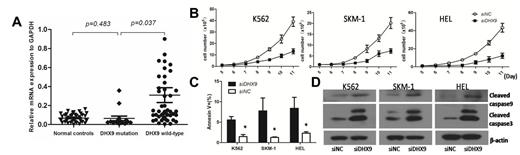
4. The quantitive PCR revealed that the patients with DHX9 mutations had lower expression of DHX9 mRNA than those without DHX9 mutations. The MDS patients had significantly higher expression of DHX9 than normal controls. No difference was observed in DHX9 expression between the MDS patients with DHX9 mutations and normal controls (Fig.2A).
5. In vitro experiments,knockdown of DHX9 induces increased cell apoptosis and inhibits cell growth in myeloid cell lines (SKM-1, K562 and HEL cells) (Fig.2B and 2C). Knockdown of DHX9 significantly enhanced the expression of apoptotic proteins (Fig.2D).
Conclusion
DHX9 mutations are novel recurrent molecular aberrations in MDS and closely related to bone marrow failure.
The distribution of DHX9 mutations and the effect of DHX9 mutations or combination with other genes mutations on patients' survival.
The distribution of DHX9 mutations and the effect of DHX9 mutations or combination with other genes mutations on patients' survival.
The mRNA expression of DHX9 in MDS and the effect of DHX9 knockdown in myeloid cell lines
The mRNA expression of DHX9 in MDS and the effect of DHX9 knockdown in myeloid cell lines
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal