Abstract
Introduction:
Tyrosine kinase inhibitors (TKIs) have dramatically improved outcome of patients with chronic myeloid leukemia (CML) and Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) with lesser incidence of serious adverse events. Recently, cases with fatal pulmonary hypertension (PH) have been sporadically documented in association with dasatinib treatment. French group reported incidence of PH diagnosed by cardiac catheterization as 0.45% (13 of 2900 patients) in the symptomatic patients treated with dasatinib. In contrast, a Korean group prospectively evaluated PH by non-invasive echocardiography in CML patients treated with dasatinib, and demonstrated 8 of 66 patients (12.1%) exhibited a significant increase in right ventricular systolic pressure, indicating subclinical PH might be more common event in dasatinib-treated patients. In this study, we prospectively examined the patients treated with three TKIs by echocardiography to clarify incidence of clinical and subclinical PH as well as factors associated with PH.
Patients and Methods:
Total of 108 patients (99 with CML, 9 with Ph+ ALL) receiving TKIs in our institutions were enrolled to this study. Forty-one patients have been on treatment with dasatinib (38%), 37 with imatinib (34%) and 30 with nilotinib (28%). Patients underwent echocardiography to evaluate values of tricuspid regurgitation pressure gradient (TRPG), which relates to severity of PH. Patients with higher values of TRPG than the upper limit (30mmHg) was suspected of PH by European Society of Cardiology criteria.
Results:
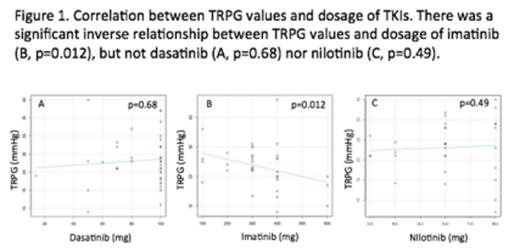
Among 108 patients, median age was 63 years old, and median duration of TKIs treatment was 26.5 months. Median daily dosage was 100 mg for dasatinib, 300 mg for imatinib, 600 mg for nilotinib groups, respectively (Table 1). In imatinib group, patients' age was significantly higher, and duration of treatment was also longer than those of the 2nd generation TKIs. Echocardiography revealed mean values of TRPG as 23.2, 23.2 and 23.1 mmHg in dasatinib, imatinib and nilotinib groups, respectively. There was no significant difference in TRPG values among 3 groups (p=0.99). We also found no relationship between TRPG values and duration of TKIs treatment in each group. Interestingly, we detected a significant inverse correlation between daily dosage of imatinib and TRPG values (p=0.012, Figure 1), while such relationship was not observed in dasatinib and nilotinib groups (p=0.68 and p=0.49). TRPG values higher than 30 mmHg were documented in 13 of 108 patients (12.0%); 5 of 41 (12.2%) in dasatinib group, 4 of 37 (10.8%) in imatinib group, and 4 of 30 (13.3%) in nilotinib group (p=0.95).
Discussion:
PH is characterized by proliferation of pulmonary vascular smooth muscle cells (SMCs). Recent reports showed that imatinib suppresses abnormal proliferation of SMCs through inhibiting platelet-derived growth factor receptors (PDGFR), resulting in improvement of PH in animal models. Clinical studies in symptomatic PH patients reported that imatinib considerably improved pulmonary hemodynamics. Of note, in our study, dosage of imatinib was significantly correlated with lower values of TRPG, suggesting imatinib possibly decreases TRPG values in a dose-dependent manner. This finding strongly supports the reports indicating imatinib as a therapeutic agent of PH. In contrast, in vitro studies have shown that dasatinib has stronger potential to inhibit PDGFR compared to imatinib; nevertheless, onsets of PH have been reported in dasanitib-treated patients, but not with imatinib nor nilotinib. Our study demonstrated the incidence of TRPG elevation as 12.0% in dasatinib group, which was consistent with Korean report (12.1%). However, there was no significant difference in TRPG values among 3 groups, indicating no apparent evidence which dasatinib treatment might be specifically associated with occurrence of PH. These results suggested that subclinical PH might be more common than expected. Careful follow-ups with echocardiography are necessary for the patients under any TKI treatments.
Patient characteristics
| . | Dasatinib group (n=41) . | Imatinib group (n=37) . | Nilotinib group (n=30) . | p . |
|---|---|---|---|---|
| Median age | 55 (17-77) | 68 (22-92) | 62.5 (24-85) | 0.0004 |
| Median dosage (mg/day) | 100 (18-100) | 300 (100-600) | 600 (300-800) | |
| Mean months from start of TKI | 68.5 (2-287) | 104.8 (2-228) | 61.3 (3-153) | 0.007 |
| Mean TRPG (mmHg) | 23.2 (9-40) | 23.2 (8-46) | 23.1 (7-35) | 0.995 |
| . | Dasatinib group (n=41) . | Imatinib group (n=37) . | Nilotinib group (n=30) . | p . |
|---|---|---|---|---|
| Median age | 55 (17-77) | 68 (22-92) | 62.5 (24-85) | 0.0004 |
| Median dosage (mg/day) | 100 (18-100) | 300 (100-600) | 600 (300-800) | |
| Mean months from start of TKI | 68.5 (2-287) | 104.8 (2-228) | 61.3 (3-153) | 0.007 |
| Mean TRPG (mmHg) | 23.2 (9-40) | 23.2 (8-46) | 23.1 (7-35) | 0.995 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal