Abstract
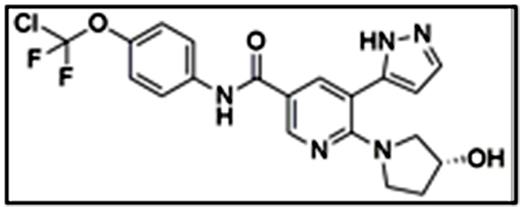
ABL001 (Fig. 1) is a first-in-class allosteric inhibitor of BCR-ABL1 that occupies the vestigial myristoyl pocket, inducing an autoinhibited kinase conformation (Sellers, et al. Proceedings of the 106th Annual Meeting of the AACR; 2015 Apr 18-22; Philadelphia, PA). Phase 1 clinical trials with single-agent ABL001 in relapsed patients with chronic or accelerated phase chronic myeloid leukemia (CML) previously treated with at least two different tyrosine kinase inhibitors (TKIs) are underway. Patients who exhibit relapsed disease associated with the presence of the T315I mutation after at least one TKI are also eligible if no other effective therapy exists. The unexplored possibility of combining an allosteric inhibitor (e.g. ABL001) with a clinically proven BCR-ABL1 TKI such as nilotinib could have important implications for maximum disease control.
Given the remote location of the myristoyl allosteric pocket relative to the catalytic site in the kinase domain of BCR-ABL1, a reasonable expectation is that ABL001 should be insulated from point mutations that impart resistance to TKIs such as nilotinib, dasatinib or ponatinib. However, pre-clinical studies demonstrate that the high level of potency exerted on native BCR-ABL1 is not uniformly maintained against a panel of clinically observed point mutations that impart resistance to non-allosteric BCR-ABL1 TKIs (Wylie, et al. Blood (2014) 124, 21, Abstract 398). For example, the T315I and F359V mutations confer substantial in vitro resistance to ABL001 in cell line experiments. The mechanistic reasons for these differences are under investigation.
We will report on: (1) Ba/F3 cell line resistance assays predictive of clinical mutation-based escape mechanisms starting from cells expressing native BCR-ABL1 or BCR-ABL1T315I, for ABL001 as a single agent and in combination with a BCR-ABL1 TKI (nilotinib, dasatinib or ponatinib) (2) profiling of ABL001 against T315I-inclusive and non-T315I compound mutations, as a single agent and in combination with a BCR-ABL1 TKI (nilotinib, dasatinib or ponatinib) and (3) Molecular Dynamics studies probing the structural reasons for the selectivity profile of ABL001.
In summary, our results to date on ABL001 suggest that single-agent ABL001 retains activity against a subset of non-T315I compound mutants. Preliminary findings indicate that the in vitro resistance profile of ABL001 is non-overlapping with that of TKIs that bind in the catalytic site. In contrast to ABL001, it is known that some myristoyl pocket binders activate rather than induce autoinhibition of BCR-ABL1. Current computational modeling efforts are focused on exploring a similar bifurcation from the perspective of native BCR-ABL1 as compared to ABL001-resistant BCR-ABL1 mutants such as T315I and F359V. Results of ongoing experiments with T315I-inclusive compound mutants will be reported. Our ultimate interest is to identify ABL001-sensitive compound mutants and make this information available to clinicians.
Druker:Millipore: Patents & Royalties; ARIAD: Research Funding; Fred Hutchinson Cancer Research Center: Research Funding; Aptose Therapeutics, Inc (formerly Lorus): Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Oregon Health & Science University: Patents & Royalties; Henry Stewart Talks: Patents & Royalties; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; McGraw Hill: Patents & Royalties; Cylene Pharmaceuticals: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; CTI Biosciences: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding; Oncotide Pharmaceuticals: Research Funding; MolecularMD: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Research Funding; Roche TCRC, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sage Bionetworks: Research Funding; AstraZeneca: Consultancy. Deininger:Ariad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal