Abstract
Background: Central nervous system (CNS) relapse in pts with aggressive non-Hodgkin lymphoma (NHL) is a generally fatal complication, with median overall survival (OS) of less than six months (Abramson et al., 2010). Several studies have identified features associated with increased risk of CNS relapse, such as extranodal (EN) sites of disease, elevated lactate dehydrogenase (LDH), presence of B symptoms, and bone marrow involvement (Bernstein et al., 2009; van Besien et al., 1998). Moreover, multivariate analyses have suggested that LDH greater than 3x upper limit of normal (ULN) is strongly associated with increased risk of death or progression among patients with aggressive NHL (Zhou et al., 2014). Despite little evidence on its true efficacy, prophylaxis (ppx) intrathecal (IT) chemotherapy, most frequently with methotrexate (MTX), is often used to among pts thought to be at high risk for CNS relapse. Data on efficacy or need for CNS prophylaxis in patients receiving infusional therapy with etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (R-EPOCH) is not available. As R-EPOCH, is being increasingly used in pts with lymphoma, we sought to evaluate the role of IT chemotherapy when used with this regimen.
Methods: We conducted a retrospective chart review analysis of patients with diffuse large B-cell lymphoma (DLBCL) who received R-EPOCH as frontline therapy, between 2005 and 2014. Use of IT ppx was at the discretion of the treating physician. We excluded patients under the age of 18, those receiving fewer than two cycles of R-EPOCH, those with CNS involvement at time of diagnosis, and those who received high-dose intravenous CNS ppx. Two-tailed Fisher's exact test was used to determine whether any of the following baseline features was associated with risk of CNS relapse: age>60, ECOG PS>1, LDH>normal and/or >3x ULN, presence of B symptoms, two or more EN sites, anatomic location of EN sites, international prognostic index (IPI) score > 1, bone marrow involvement, and HIV infection. In order to determine whether IT ppx was associated with any improvement in CNS and/or systemic control of disease, we compared, using Kaplan-Meier survival curves with log-rank analyses, the patterns of overall survival (OS), progression-free survival (PFS), and freedom from CNS progression (FFCP, in which death is not counted as an event) between patients receiving IT ppx and those not receiving it.
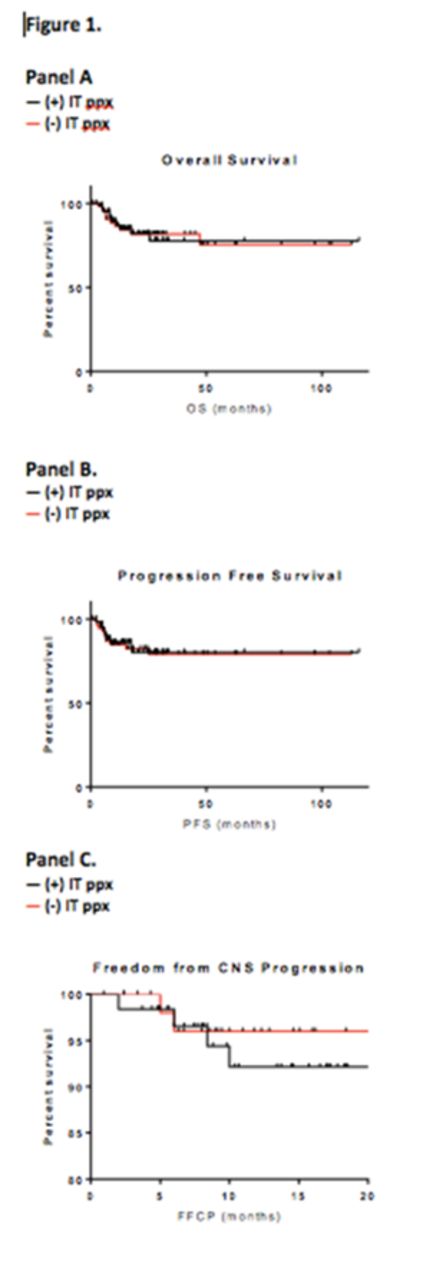
Results: We identified 117 patients for analysis. Median age was 53 (range 19-80). 26% had ECOG PS>1; 76% had LDH above upper limit of normal (ULN). 38% had two or more EN sites of disease, and 68% had stage III-IV disease. 62 patients received IT ppx, and 55 did not. Of those receiving IT ppx, 95% received MTX, with the remainder receiving cytarabine. Those receiving IT ppx were more likely to have >1 EN site of disease, and IPI score >1 (Table 1). A total of seven had observed CNS relapse, occurring at a median of 6 months from time of NHL diagnosis (range 2-24 months). At a median follow up of 18 months, the 24-month PFS and OS were 80% and 83%, respectively. Median PFS and OS were not reached. The only factors associated with increased risk of CNS relapse were genitourinary EN disease and LDH >3x ULN (Table 2). There were no significant differences in OS, PFS, or FFCP among patients who did and did not receive CNS prophylaxis (Figure 1, panels A-C).
Conclusions: The risk of CNS progression among DLBCL patients receiving R-EPOCH was similar to previous reports with R-CHOP, at 6%. GU location of EN disease and LDH >3xULN were associated with increased risk of CNS relapse. IT ppx was not associated with improved outcomes. Despite the common use of IT PPX in pts treated with R-EPOCH, our data suggest that this practice might not impact CNS progression and/or relapse, though randomized studies would be needed to answer this. Such studies are warranted in order to better determine what factors are associated with CNS progression, and whom, if anyone, may benefit from IT ppx.
Nabhan:Celgene Corporation: Honoraria, Research Funding. Petrich:Seattle Genetics: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal