Abstract
Despite of great improvement of treatment outcome by ABL kinase inhibitors, Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is still intractable disease, and novel therapeutic agents are anticipated. We recently developed a high-throughput drug screening system using patient-derived xenograft (PDX) cells. PDX cells highly maintain phenotypes of primary malignant cells, such as heterogeneity of cancer cells, slower growth rate than cell lines, and microenvironment dependency. Drug screening by PDX cells can pick up anti-tumor reagents with new mechanisms that were overlooked by conventional cell-line based screenings. Here, we discovered verteporfin, an approved drug for macular degeneration, as a candidate for novel therapeutic agent of Ph+ ALL.
We established PDX of 3 Ph+ ALL patients (PhLO, PhLK, and PhLH) by transplanting primary Ph+ ALL cells into NOD/SCID/IL-2Rgnull (NOG) mice. We developed a high-throughput drug screening system using one of these PDX cells (PDX screening) and screened the library of 3440 compounds containing approved drugs and pharmacologically active reagents. The profile of drugs selected by PDX screening was quite different from that by the screening using Ph+ ALL cell line (Cell line screening). Verteporfin was selected by PDX screening, whereas it did not demonstrate very high anti-leukemic effect in Cell line screening.
We confirmed the anti-leukemic effect of verteporfin ex vivo using the 3 PDX cells and ALL-1, the cell line used in the Cell line screening. All 3 PDX cells were more sensitive to verteporfin than ALL-1 (EC50: PhLO cells, 228 nM; PhLK cells, 1.8 µM; PhLH cells; 395 nM; ALL-1, 3.93 µM). In addition, combined use of verteporfin and dasatinib, an ABL kinase inhibitor used for the treatment of Ph+ ALL, showed synergistic growth suppression of PhLO cells. Combination index (CI) values calculated by combination index algorism were less than 1.0 in most combinations of 16 data points (CI mean, 0.73; CI range, 0.28-1.34).
Furthermore, the mechanism of action of verteporfin was intensively investigated. In combination with red light irradiation, verteporfin induces apoptosis of tumor cells through production of reactive oxygen species (ROS), which is known as photodynamic therapy. We revealed that verteporfin produced ROS light-independently in PhLO cells and induced their apoptosis. Verteporfin-induced apoptosis was inhibited by the addition of reduced glutathione to the culture medium, suggesting the large involvement of ROS production in the verteporfin-induced apoptosis.
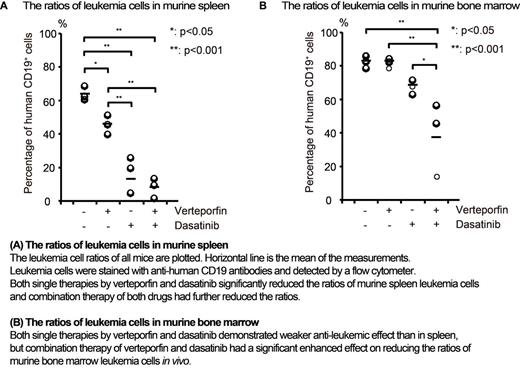
Finally, we assessed the in vivo effect of verteporfin. NOG mice transplanted with PhLO cells were treated with vehicle, verteporfin (140mg/kg/day), dasatinib (20mg/kg/day), or both of them from day 22 to day 28. Dasatinib was intraperitoneally injected and verteporfin was administered by continuous subcutaneous injection using osmotic pumps because of its very short half-life. We measured the ratios of leukemic PDX cells in bone marrow and spleen on day 28. Both single therapies by verteporfin and dasatinib significantly reduced the leukemia cell ratios in spleen and the combination therapy of them further reduced leukemia cells in spleen (Figure A). In bone marrow, both single therapies demonstrated weaker anti-leukemic effect than in spleen, but the combination therapy showed significantly enhanced effect, indicating the synergistic effect between them in vivo (Figure B). These results indicate the promisingness of verteporfin as a new anti-leukemic reagent and PDX screening as a new strategy for the development of anti-cancer drug.
Naoe:Pfizer Inc.: Research Funding; Toyama Chemical Co.,LTD.: Research Funding; Otsuka Pharmaceutical Co.,Ltd.: Patents & Royalties, Research Funding; Nippon Boehringer Ingelheim Co., Ltd.: Research Funding; Kyowa-Hakko Kirin Co.,Ltd.: Patents & Royalties, Research Funding; Fujifilm Corporation: Patents & Royalties, Research Funding; Chugai Pharmaceutical Co.,LTD: Patents & Royalties; Celgene K.K.: Research Funding; Astellas Pharma Inc.: Research Funding. Kiyoi:Yakult Honsha Co.,Ltd.: Research Funding; Novartis Pharma K.K.: Research Funding; MSD K.K.: Research Funding; Eisai Co., Ltd.: Research Funding; Alexion Pharmaceuticals: Research Funding; FUJIFILM Corporation: Patents & Royalties, Research Funding; Nippon Boehringer Ingelheim Co., Ltd.: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; FUJIFILM RI Pharma Co.,Ltd.: Research Funding; Teijin Ltd.: Research Funding; Japan Blood Products Organization: Research Funding; Astellas Pharma Inc.: Consultancy, Research Funding; Taisho Toyama Pharmaceutical Co., Ltd.: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding; Pfizer Inc.: Research Funding; Mochida Pharmaceutical Co., Ltd.: Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal