Abstract
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA180795, CA180791, CA189859, CA180790, CA180853, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Background: Relapse after achieving a complete remission (CR) in AML portends for a poor prognosis and allogeneic transplant after achieving a second remission is the only chance for cure. Patients who undergo a transplant have a 30-40% chance of long term survival. This is a follow-up of a report published 10 years ago (Rowe JM, ASH 2005, abstract 546) and includes contemporary studies and longer follow-up. The study examines the long term overall survival (OS) of AML patients who relapsed after achieving first CR in 9 successive ECOG-ACRIN trials for newly-diagnosed AML patients (E3483, E3489, PC486, E3993, E4995, E1490, E3997, E1900 and E3999) from March 1984 to November 2008.
Methods:
OS was defined as time from first relapse to death from any cause. Kaplan-Meier estimates were used to estimate the event-time distributions for OS. Multivariate model stratified on protocol and treatment were used to examine whether the following factors are prognostic for OS from relapse: age, gender, cytogenetic risk, ECOG performance status, WBC, platelets, hemoglobin, marrow blasts, peripheral blasts, and duration of CR. All P values were based on 2-sided tests.
Results:
A total of 3160 patients were enrolled in the 9 studies. The median follow-up on patients still alive was 10.0 years. Among those 3160 patients enrolled, 1864 (58.9%) achieved first CR of which 1086 (58.2%) had documented relapse.
The median age at diagnosis of the relapsed patients was 50 (range: 16-84) and 50.6% were males. Fifty percent of the patients had reliable cytogenetic results. Of those, 13.5% had favorable cytogenetics, 55.8% - intermediate and 30.6% had unfavorable baseline results.
The median OS from relapse was 0.5 years. The 2- and 5-year OS were 16(±1)% and 10(±1)%, respectively. This is true in even the most contemporary studies (E1900 and E3999) with median OS of 0.6 and 0.4 years, respectively.
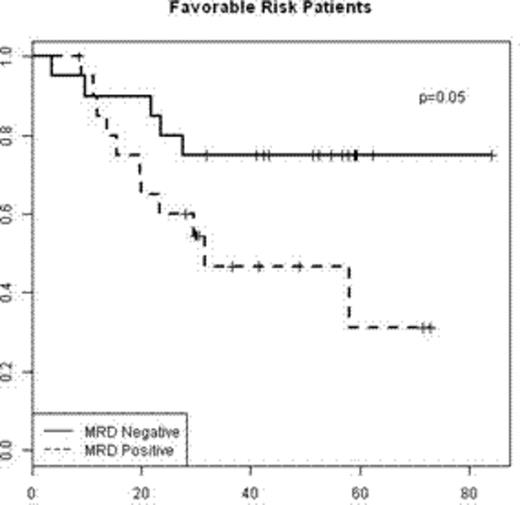
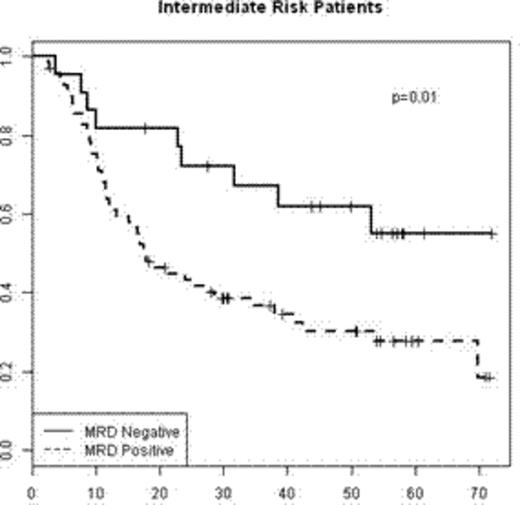
By age stratification (< or ≥ 55), 5-year OS was 13(±1)% and 5(±1)%, respectively (figure 1). Among patients<55, those with unfavorable cytogenetics had the poorest prognosis (median OS of 0.4 years and 5-year OS of 6(±3)%. Those with favorable and intermediate cytogenetics had similar OS with 0.7 and 0.6 years median OS and 5-year OS of 16(±5)% and 17(±3)%, respectively (figure 2).
Multivariate analysis was perfomed on 517 patients who had enough baseline information, including cytogenetics. Factors that were significantly associated with OS from relapse included: age (p<0.001), ECOG performance status (p=0.04), hemoglobin (p=0.03), cytogenetics (p=0.045) at baseline and duration of CR (p<0.001).
Conclusions: The short- and long-term OS of AML patients post-relapse is dismal (<10%). Although age<55 and favorable cytogenetics at diagnosis are relatively good prognostic factors, the general survival of even these patients is very poor. Disappointingly, these data are also applicable to the most contemporary studies. Long-term survival was possible only in the minority of patients who survived the relapse, achieved a second CR and then successfully underwent an allogeneic transplant. These data are crucial when considering post-remission strategy, and suggest that offering a therapy most likely to lead to cure in CR1 is the preferred option.
Probability of OS from relapse by cytogenetic risk for patients age <55
Douer:Gilead: Consultancy. Rowe:BioSight Ltd.: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx Ltd.: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal