Abstract
Prothrombin, like other vitamin K-dependent coagulation factors, undergoes γ-carboxylation of its Gla domain, a posttranslational modification critical for membrane binding. In patients on anticoagulant treatment with warfarin, the INR has historically been correlated with the degree of des-gamma-carboxy-prothrombin (DCP or PIVKA-II). PIVKA-II can be measured readily and used as a marker for vitamin K deficiency or warfarin therapy and is thought to be useful in detecting subclinical disease. Long-standing dogma suggests prothrombin γ-carboxylation is necessary for prothrombin membrane binding facilitating engagement with prothrombinase leading to rapid thrombin generation and effective hemostasis. However, recent studies indicate that despite an inability to bind membranes, uncarboxylated (desGla) full-length prothrombin demonstrated an unexpected modest decrease in the rate of thrombin generation (J Biol Chem 2013, 288:27789-800). Thus, it is possible loss of prothrombin γ-carboxylation, and thereby membrane binding, is far less significant for prothrombin activation than previously appreciated. Instead warfarin's effect on other coagulation factors (FX, FIX, and FVII) may be the primary causative determinant impairing hemostasis in these anticoagulated patients.
To test these ideas, we first analyzed thrombin generation using recombinant full-length fully carboxylated and desGla prothrombin in vitro. Human prothrombin deficient plasma (Factor II activity <4%) was reconstituted to normal levels (100 μg/mL) with desGla or carboxylated prothrombin. DesGla prothrombin generated approximately half the amount of thrombin observed in carboxylated prothrombin plasma and normal human plasma controls.
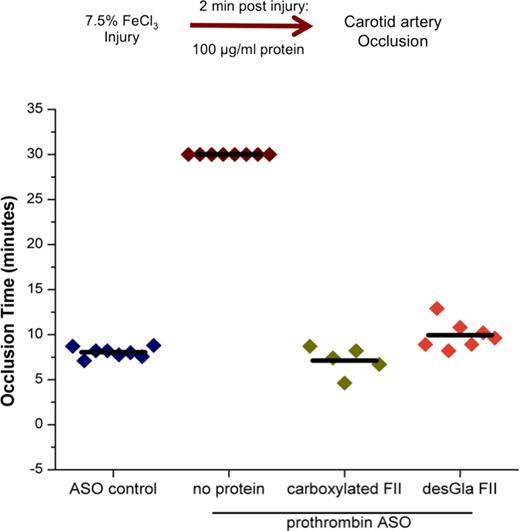
We next analyzed full-length desGla prothrombin's in vivo hemostatic function. A prothrombin anti-sense oligonucleotide (ASO) was administered to hemostatically normal mice to knock down endogenous murine prothrombin expression (<0.1-1μg/mL, 0.1-1%) and confirmed by ELISA analysis. Hemostasis was analyzed by the ferric chloride (FeCl3) carotid artery injury model. In mice treated with an ASO control, vessel occlusion occurred at approximately 8 minutes while mice treated with the prothrombin ASO did not clot during the 30-minute post injury observation period. In additional experiments two minutes following injury, prothrombin ASO treated mice were administered either carboxylated or desGla recombinant prothrombin to restore plasma concentrations to the normal range (100 μg/mL). Remarkably, administration of either desGla or carboxylated prothrombin restored vessel occlusion to ASO control findings, with minimal variability observed between desGla and carboxylated prothrombin treated mice (Figure 1).
Warfarin treatment results in impaired prothrombin γ-carboxylation. However, if prothrombin γ-carboxylation, is, in fact, not necessary for prothrombin activation, fully carboxylated Factor Xa (FXa) should reverse the effects of warfarin by efficiently activating the un/under-carboxylated prothrombin thereby bypassing the other warfarin-affected factors. To study this, we used a "zymogen-like" factor Xa (FXaI16L) molecule previously developed by our group (Nat. Biotech 2011, 29:1028-33) that has a greater half-life than the wild-type protein. In thrombin generation assays, addition of 1nM FXaI16L to plasma from patients anticoagulated with warfarin, irrespective on INR (2.8, 4,4 7.1), resulted in thrombin generation comparable to that of normal human plasma. Importantly, similar results were obtained in vivo in warfarin-anticoagulated mice (INR 2-3). Administration of 3 mg/kg FXaI16L to 8 out of 8 warfarin mice corrected the time to carotid artery occlusion in the FeCl3 injury model.
In two separate in vitro and in vivo model systems, we demonstrated that prothrombin membrane binding is not absolutely required for thrombin generation. Thrombin is unique among the coagulation serine proteases in that it does not have a Gla domain once fully processed by prothrombinase; thus, the absence of a Gla domain in the protease (thrombin) may explain the lack of a requirement for membrane binding by the zymogen (prothrombin) precursor. Our findings may also have clinical relevance, since they suggest that FXa (or a variant) could be used as a novel warfarin bypass strategy to rapidly achieve hemostasis in the setting of warfarin anticoagulation.
Greene:Baxter: Research Funding. Camire:Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Patents & Royalties, Research Funding; NovoNordisk: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal