Abstract
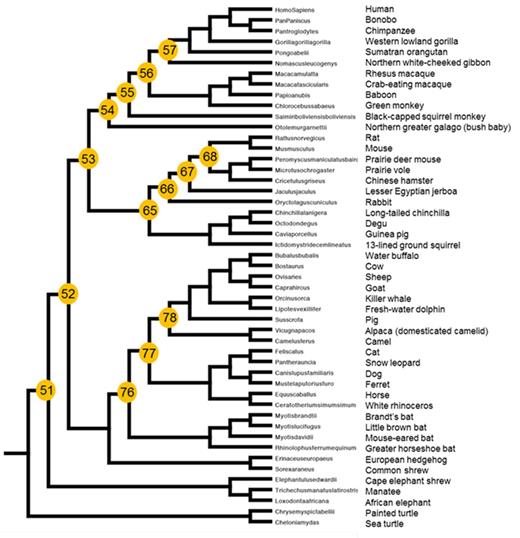
The development of transformative hemophilia A therapeutics has been hindered by the size, instability, immunogenicity and biosynthetic inefficiency of coagulation factor VIII (FVIII). Through the study of FVIII orthologs from existing vertebrate species, we discovered unique molecular, cellular and biochemical properties that can overcome the limitations of human FVIII. This approach facilitated the development of recombinant porcine FVIII for acquired hemophilia A and has enabled low resolution mapping and bioengineering of functional sequence determinants into human FVIII. To further extend this bioengineering approach, we employed a novel methodology termed ancestral protein reconstruction that provides certain advantages over 'rational design' approaches including a priori confidence that each ancestral FVIII is hemostatically functional. First, a mammalian FVIII phylogenetic tree with corresponding ancestral node (An) sequences was constructed through Bayesian inference using both DNA and amino acid-based models in PAML Version 4.1 (Figure 1). The limited availability of non-mammalian sequences precluded accurate ancestor prediction outside of this class. Initially, we selected 14 An-FVIII sequences for reconstruction and subsequent molecular, cellular, biochemical and immunological characterization. Each An-FVIII displayed activity in coagulation assays utilizing human hemophilia A plasma as a substrate thus demonstrating evolutionary mammalian compatibility. Infusion of highly purified preparations of several An-FVIIIs into hemophilia A mice also corrected the bleeding phenotype following a tail transection bleeding challenge confirming in vivo functionality. To study biosynthetic efficiency, secreted FVIII activity and mRNA transcript levels were analyzed following transfection of An-FVIII plasmids into HEK293 and BHK-M cell lines. An-53, common ancestor to rodents and primates, and An-68, ancestor to a subset of current rodents, displayed the highest FVIII biosynthetic efficiencies that were 12 and 15 fold greater than human FVIII, respectively (P = 0.002; Mann Whitney U test). These two An-FVIII sequences share 95 and 87% amino acid identity to human FVIII, respectively. In contrast, intermediate ancestors between An-53 and human FVIII, designated An-55, -56 and -57, do not display enhanced biosynthetic efficiency suggesting that the functional sequence determinant of high expression was lost during primate evolution. Predicting that high expression ancestral FVIIIs would be enabling to gene therapy approaches, An-53, An-68 and human FVIII cDNAs were placed in an AAV expression cassette under the control of a potent liver-specific promoter and the resulting plasmid DNA was infused hydrodynamically into hemophilia A mice. An-53 and An-68, but not human FVIII vector treated animals, achieved sustained, therapeutic plasma FVIII activity levels over 4 weeks (0.1 - 0.6 IU/ml versus <0.01 IU/ml, respectively). Recombinant An-FVIIIs were expressed, purified and biochemically characterized by SDS-PAGE, specific activity, decay following thrombin activation and inhibitor recognition. Early mammalian and all primate lineage thrombin-activated An-FVIII(a) displayed half-lives between 1.5 - 2.2 min that were not distinguishable from human FVIII. We have shown previously that modern murine, porcine, and ovine FVIIIa display significantly longer half-lives and thus this property may have evolved under positive selection. Supporting this conclusion, An-68 and An-78 display prolonged half-lives of 16 and 7 min, respectively. Lastly, the immune recognition of An-FVIIIs by a panel of A2 and C2 domain targeting inhibitory murine monoclonal antibodies as well as hemophilia A inhibitor patient plasmas was examined and many examples of reduced reactivity were revealed, which may enable the development of less immunogenic FVIII products. Herein, we report molecular discoveries that enhance our understanding of FVIII structure/function and provide a blueprint for bioengineering novel FVIII molecules with enhanced properties. These studies also show 'proof of concept' for ancestral protein reconstruction as a powerful approach to studying the biochemistry, molecular biology and evolution of the vertebrate coagulation system, which should enable identification of other new hematological drug targets and candidate biotherapeutics.
Spencer:Expression Therapeutics: Equity Ownership. Doering:Expression Therapeutics: Equity Ownership; Bayer Healthcare: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal