Abstract
INTRODUCTION
The cohesin complex is a multimeric protein complex reported to have putative roles in post-replicative DNA repair, sister chromatids cohesion and transcriptional regulation. Somatic mutations in the genes that constitute the cohesin complex (NIPBL, SMC1A, SMC3, and HDAC8) give rise to the debilitating disease, Cornelia de Lange Syndrome. The cohesin genes are mutated in several cancers, the highest incidence reported in bladder cancer followed by myeloid cancers such as myelodysplastic syndrome (MDS). Inhibition of PARP (Poly ADP Ribose Polymerase) activity has been shown to selectively target AML patients with DNA repair defects (Gaymes et al 2009). Discovery of mutations that confer sensitivity to PARP inhibitors have the potential to identify candidates for therapeutic intervention.
Mutation in genes involved in epigenetic regulation and RNA splicing account for 70% of the mutations reported in MDS. Mutations in cohesion complex have been reported, but have not been systematically studied in a large and uniformly treated patient cohort. To characterise still further the mutations that shape the MDS phenotype, 154 MDS patients that had been screened for myeloid gene mutations using the 22 gene panel (Syed et al 2013) were re-screened for mutations in four of the cohesin complex genes (SMC1A, SMC3, RAD21 and STAG2). The functional consequences of defective cohesin were also studied using shRNA silencing.
METHODOLOGY
Nextera sequencing was used to sequence all the coding exons of SMC1A, SMC3 and RAD21 genes, whilst STAG2 was sequenced using the Roche 454 platform. To further elucidate the role of cohesin complex in MDS, lentiviral shRNA silencing of STAG2 was achieved in the AML cell line, U937 and effects of knockdown was subsequently determined through functional assays.
RESULTS
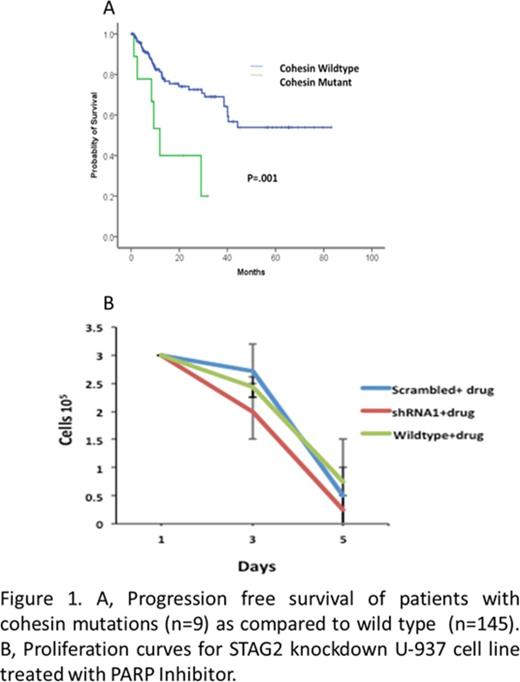
Sequencing of the cohesin complex genes revealed mutations in 6% of MDS patients of which 4% had STAG2, 1% had SMC3 and0.6% patients had SMC1A mutations. None of the patients studied had a RAD21 mutation. All detected mutations were confirmed by independent PCR and Sanger Sequencing. For STAG2 mutations, a constitutional source of DNA was also used to confirm the mutations, (skin biopsy (n=2), T cells (n=3). 5/6of the STAG2 mutationswere nonsense with an average allelic burden of 33% indicative of a heterozygous mutation. Mutations in SMC3 were reported in two patients; a frameshift mutation found in the coiled coiled domain and a substitution in the 3' splice acceptor site. The average allelic burden for both the mutations was 20%. A missense mutation was identified in SMC1A in one patient with an allelic burden of 13%. (3/9) patients mutations in STAG2 gene coexisted with SRSF2 and ASXL1 mutations. The patients with cohesion mutations had a significantly lower progression free survival as compared to patients without mutation (Figure 1A)
Lentiviral shRNA silencing of 75% and 66% for STAG2 was achieved in U937 cells using two shRNAs, confirmed both by q-PCR and Western blot. shRNA silencing of STAG2 also resulted in reduced expression of partner cohesin sub-unit, RAD21 confirmed by western blot. Strikingly, treatment of shRNA silenced U937 with PARP (Poly-ADP-Ribose Polymerase) inhibitors resulted in 28% cytotoxic reduction compared to scrambled control (Figure 1B). Furthermore, cytotoxicity was independent of Homologous Recombination DNA repair competency as recruitment of RAD51 to damaged sites was not altered in shRNA silenced STAG2 U937 cells upon challenge with PARP inhibitors.
CONCLUSION
In this study, cohesin complex mutations were found in 6% of MDS patients with STAG2 being the most prevalent. We also report the co-existence of cohesin mutations with mutations in ASXL1 and SRSF2. The cohesin mutant allelic burden in these patients was lower compared to ASXL1 and SRSF2 mutations suggesting that cohesin mutations co-exist as passenger mutations alongside mutations that drive clonal evolution like ASXL1 as reported previously (Thota et al 2014). The observation that loss of STAG2 confers PARP inhibitor sensitivity is of major clinical importance. Identification of a cohort of MDS/AML patients with cohesin mutations would signify a major development in the identification of candidates for PARP inhibitor therapy.
Kulasekararaj:Alexion: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal