Abstract
BACKGROUND: Despite high rates of initial response to immunomodulatory agents and proteasome inhibitors, the vast majority patients living with multiple myeloma (MM) will ultimately die of progressive disease that is a function of persistent MM cell clones. The radiosensitivity of malignant plasma cells is well documented in clinical settings, and we have previously reported the therapeutic efficacy of a streptavidin-biotin (SAB) pretargeted radioimmunotherapy (PRIT) system delivering the β-emitter 90Y to CD38 antigen targets (Green, et al. Cancer Res. 2014). While SAB-PRIT is capable of disease eradication in mouse models, SA immunogenicity and interference by endogenous biotin may complicate clinical translation of these findings into human trials.
METHODS: To prospectively address these potential limitations and to further enhance therapeutic efficacy of PRIT, we engineered an anti-human CD38 xanti-chelated radiometal fusion protein (FP) specifically designed to trap second-step radiolabeled ligand (Y-DOTA) using a very high affinity anti-Y-DOTA scFv (C825). The bispecific anti-CD38 FP construct (028Fc-C825) expressed in CHO cells was affinity purified, assessed by gel filtration chromatography and binding specificity to both CD38 and Y-DOTA was confirmed. In vivo experiments showed the absence of toxicity of the bispecific construct at a dose range of 0.14-2.8 nmol per mouse (n=5/group). Biodistribution studies were performed in athymic nude mice (n=5/group) bearing s.c. luciferase transduced NCI-H929Luc human MM xenograft tumors. Animals received 2.8 nmol (420 µg) of either 028-Fc-C825 (anti-CD38 FP) or an irrelevant matched negative control bi-specific FP 2H7-Fc-C825 (anti-CD20 FP) with the same modular IgG-scFv structure (NCI-H929Luc are CD20 negative). The bispecific FP was administered 23 hours prior to a synthetic DOTAY-Dextran clearing agent (CA; 5 µg) and 24 hours prior to administration of trace labeled 90Y-DOTA (2 µg). In therapy studies, athymic nude mice (n=10/group) bearing s.c. NCI-H929Luc human MM xenograft tumors received 2.8 nmol of either 028-Fc-C825 (anti-CD38 FP) or 2H7-Fc-C825 (anti-CD20 FP) 23 hours prior to synthetic DOTAY-Dextran CA and 24 hrs prior to 90Y-DOTA (2 µg; 1200 µCi per mouse). An activity range of 800 µCi to 1200 µCi was previously identified as optimal in a MM xenograft model using the high energy beta particle emitter 90Y (t1/2 = 64 hours) [Green, et al. Cancer Res. 2014].
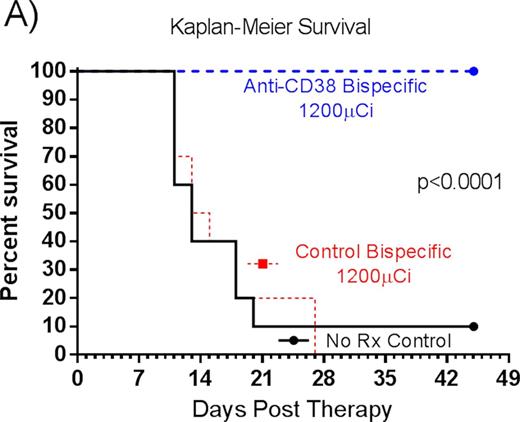
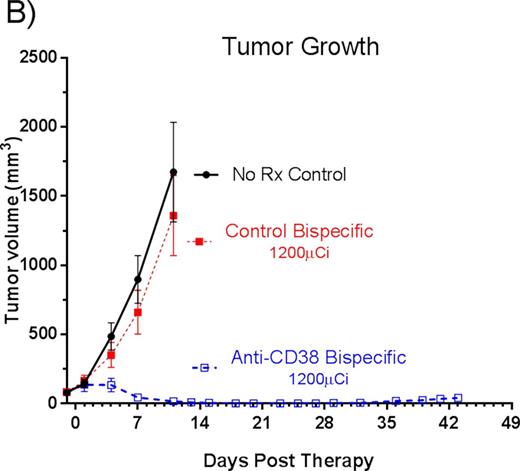
RESULTS: Selective tumor targeting of 90Y ligand to CD38+ xenografts was observed following administration of the bispecific anti-CD38 FP. Blood, tumor and nonspecific organ uptakes of 028-Fc-C825 demonstrated tumor-to-normal organ absorbed ratios that were 15:1; 17:1; 9:1; and 13:1, respectively for blood, lung, liver and kidney; compared to 0.7:1; 1:1; 1:1 and 0.5:1, respectively, in mice pretargeted with 2H7-Fc-C825 (control). The bispecific anti-CD38 FP also resulted in tumor-to-normal organ ratios of absorbed radioactivity that were superior to those measured in animals receiving anti-CD38 SAB-PRIT (OKT10-SA chemical conjugate) in the same study (5:1; 8:1; 5:1 and 2:1 for blood, lung, liver and kidney respectively). In therapy studies, no animal in any group lost >7% of initial body weight and by day 11 anti-CD38 bispecific FP treated animals were 102.4% ± 3.4% of baseline weight. All mice in the control bispecific FP group experienced exponential MM tumor growth and 100% of these control animals required euthanasia within 27 days. All mice pretargeted with 028-Fc-C825 (anti-CD38 bispecific FP) demonstrated tumor response by day 6. After 45 days, 100% of the anti-CD38 bispecific FP treated animals remained alive (Figure A) and objective remissions were observed within 18 days in 100% of the mice treated with 028-Fc-C825 followed by 1200 µCi of 90Y-DOTA, including 90% complete remissions (no detectable tumor in 028Fc-C825 treated mice) compared to tumors that were 2040 ± 1389% of initial tumor volume [p<0.0001, Student's t-test] in untreated control animals by day 12 (Figure B).
CONCLUSION: Biodistribution studies demonstrate MM tumor specific targeting after anti-CD38 xanti-chelated radiometal FP with minimal levels of yttrium-90 radioactivity detected in normal organs. Tumor responses are very encouraging in this MM xenograft model and results support clinical evaluation of bispecific anti-CD38 FP PRIT in MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal