Abstract
Background:MYC and BCL2 are critical driver genes for non-Hodgkin lymphoma including diffuse large B-cell lymphoma (DLBCL). However, the clinical impact of MYC and BCL2 genetic alterations, apart from translocations, has not been comprehensively investigated using high-resolution genetic assays, such as next generation sequencing and high-resolution SNP arrays. Moreover, correlations with cell of origin (COO) subtype, determined by gene expression profiling, have not been widely studied in a large homogeneously treated cohort. We determined the frequency and clinical impact of MYC and BCL2 genetic aberrations in DLBCL in a large population-based cohort uniformly treated with R-CHOP.
Methods: We analyzed 347 newly diagnosed de novo DLBCL cases that were treated with R-CHOP in BC. Comprehensive clinical annotation was available through the BCCA Lymphoid Cancer Database. Deep targeted re-sequencing of the coding exons of MYC and BCL2 was performed using a Truseq Custom Amplicon assay (Illumina) on the Miseq platform. High-resolution copy number analyses were performed using Affymetrix SNP 6.0 arrays. Immunohistochemical staining and break-apart FISH assays for MYC and BCL2 were performed on tissue microarrays (n=332). Dual positivity for MYC (cut off; 40%) and BCL2 (cut off; 50%) proteins identified a dual protein expresser (DPE) phenotype. COO classification was achieved using the Lymph2Cx assay based on NanoString technology in 299 patients and the Hans algorithm in 32 cases with low tumor content (<40%).
Results: COO determination revealed 193 cases to be GCB subtype, 107 cases ABC/non-GCB and 30 were unclassifiable. Using next generation sequencing, 310 SNVs were detected in MYC (29/347; 8% of cases) and BCL2 (88/347; 25% of cases), with mean redundant coverage depths of 633-fold. All MYC and 98% of BCL2 mutations were misssense mutations. Analysis of copy number alterations using GISTIC 2.0 revealed significant focal gain/amplification (gain/amp) peaks affecting 8q24, including the MYC locus (68/341; 20% of cases) and 18q21, including the BCL2 locus (82/341; 24% of cases). MYC and BCL2 translocations were detected in 38/283 (13%) and 90/300 (30%) of tumors, respectively.
MYC gain/amp, BCL2 mutation, BCL2 translocations and double MYC/BCL2 translocation (DHIT) were seen significantly more often in GCB-DLBCL (26% vs 10%, p=0.001; 35% vs 11%, p<0.001; 44% vs 6%, p<0.001; and 13% vs 0%, p<0.0001, respectively), and BCL2 gain/amp was observed more commonly in ABC-DLBCL (44% vs 12%, p<0.001). MYC translocations were significantly associated with MYC protein expression in ABC, GCB and all cases (p<0.0001). On the other hand, BCL2 protein expression was significantly associated with BCL2 mutation and translocation in GCB-DLBCL (both, p<0.0001), and BCL2 gain/amp in ABC-DLBCL (p=0.0037).
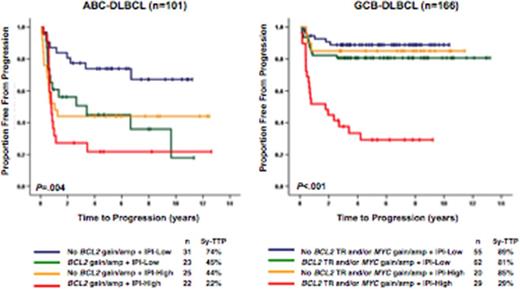
With a median follow up of 6.5 years for living patients, the presence of MYC translocation, BCL2 gain/amp and BCL2 mutation were associated with an inferior 5y-time to progression (TTP, 53% vs 30%, p=0.019; 60% vs 32%, p=0.009 and 52% vs 17%, p=0.026, respectively) in ABC subtype. In GCB subtype, BCL2 translocation, MYC gain/amp and MYC translocation were associated with an inferior 5y-TTP (85% vs 62%, p=0.001; 80% vs 63%, p=0.011; and 79% vs 61%, p=0.013, respectively). In a multivariate Cox model of TTP including IPI and DPE, BCL2 gain/amp remained prognostic (HR=2.4 [1.3-4.5], p=0.008) independent of IPI and DPE in ABC-DLBCL. In GCB-DLBCL, BCL2 translocation and/or MYC gain/amp showed strong prognostic value (HR=3.0 [1.4-6.4], p=0.006) independent of IPI and DPE. In the IPI high risk group (IPI=3-5), the presence of a BCL2 translocation and/or MYC gain/amp defined a remarkably poor outcome group in GCB-DLBCL (5y-TTP; 29%). Similar poor outcome was observed in ABC-DLBCL cases that harbored BCL2 gain/amp (5y-TTP; 22%).
Conclusions: The DHIT genotype was only seen in GCB-DLBCL. High-resolution genomic assays identified extremely poor prognostic groups within each COO subtype on the basis of MYC and BCL2 genetic status in a large uniformly R-CHOP-treated population-based cohort of DLBCL.
TTP according to MYC/BCL2 genetic alterations with IPI in ABC-DLBC (n=101) and GCB-DLBCL patients (n=166) treated with R-CHOP.
TTP according to MYC/BCL2 genetic alterations with IPI in ABC-DLBC (n=101) and GCB-DLBCL patients (n=166) treated with R-CHOP.
Savage:Seattle Genetics: Honoraria, Speakers Bureau; BMS: Honoraria; Infinity: Honoraria; Roche: Other: Institutional research funding. Connors:Roche: Research Funding; Seattle Genetics: Research Funding. Scott:Celgene: Consultancy, Honoraria; NanoString: Patents & Royalties: Inventor on a patent that NanoString has licensed.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal