Abstract
Background: Thrombopoietin receptor agonists (TPO-RA) eg eltrombopag (Epag), are highly effective treatments of ITP as demonstrated in multiple randomized studies. Studies have shown effective and safe long-term use; toxicity appears tolerable with thromboembolism the most common serious adverse event. An important question is if there will be an indefinite long-term need for treatment with these agents once initiated. The aim of this study is to taper eltrombopag in a single arm study to determine how many and which patients will be able to discontinue treatment over time. It specifically targets patients with adequate counts ie 50-100,000/uL, not waiting for patients whose counts spontaneously increase to high levels and thus indicate that they are being over-dosed.
Methods: All patients on eltrombopag at doses of 75 mg daily or less for at least 4 months were offered study entry; only one refused. Initially the study tapered Epag rapidly but then changed to slow tapering over up to 2 years. Patients whose platelet counts were >50,000/uL were tapered at 4 week intervals in 10-20% dose increments. Tapering could be postponed for clinical reasons ie impending procedure or trip, infection skewing count, sports or other physical activity, etc. Analysis was descriptive and t test comparisons used to assess variables associated with successful tapering.
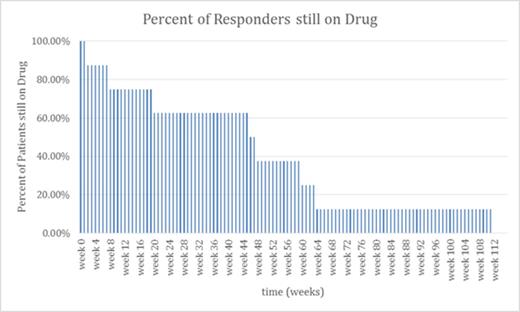
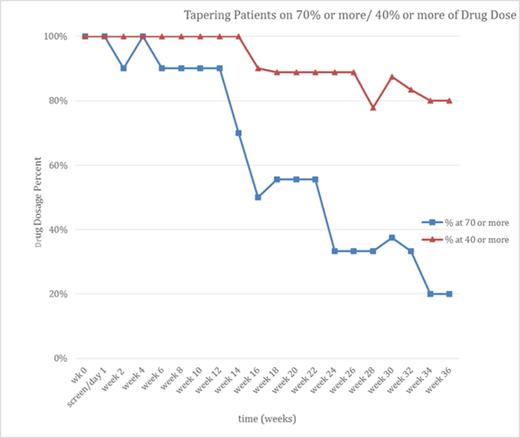
Results: Patients were divided into 3 groups: 10 responders (those able to discontinue eltrombopag completely within 2 years, fig 1A), 10 tapering (those still tapering who have reduced their medication but not discontinued it, fig 1B), and 12 non-responders (those who tried and failed to discontinue within 2 years). To complete being a responder required >12 weeks without any ITP treatment, platelet count > 30,000/uL and 20,000/uL more than baseline after the last dose of eltrombopag. Eight of the 10 responders have remained off therapy for more than 1 year with platelet counts consistently > 50,000/uL. No medication was provided in the study. No serious AEs occurred and no serious bleeding events were seen. Fig 1a illustrates the discontinuation of eltrombopag treatment in responders: 4 discontinued within 8 weeks while the longest discontinuation required a full 2 years. Fig 1b shows patients who are tapering eltrombopag but have not reached 2 years by indicating how many are on <70% and <40% of their starting doses; it shows that tapering is typically a slow process but that at least dose reduction can be achieved in many patients even if they do not discontinue their eltrombopag.
Table 1 compares means and medians of the 3 groups for a number of clinical variables. While the numbers are small and the results preliminary, it surprisingly shows that patients who have been on eltrombopag longer prior to tapering and those who have received more treatments are more likely to successfully discontinue eltrombopag. In contrast, non-responders failed splenectomy and rituximab more often than did those tapering and responders. Finally AIPF at initiation of study did not predict a successful taper.
Summary: A substantial fraction of patients with chronic ITP will be able to successfully taper off eltrombopag; the exact number (somewhere between 10 and 20 of the 32 patients) depends upon the group still tapering. The tapering schedule selected is slow and requires regular monitoring and individualization/flexibility. Even if patients do not fully taper off eltrombopag, they may be able to substantially reduce their dose. We speculate that tapering eltrombopag as outlined here may reduce the toxicity and costs of long-term treatment without diminishing hemostatic efficacy.
| Comparison Table (Responders, Tapering, Non-Responders) . | Responders (n=10) . | Tapering (n=10) . | Non-Responders (n=12) . |
|---|---|---|---|
| Age (years) (median/average) | 29/37.4 | 11/25.70 | 21/30.58 |
| Gender (m/f) | 3/7 | 6/4 | 5/7 |
| Duration ITP (years) (median/average) | 8.75/11.95 | 5.5/17.45 | 7/8.25 |
| Duration of TPO-RA Therapy (years) (median/average) | 5/4.2 | 5.5/7.45 | 2.5/3.38 |
| # of prior ITP treatments (median/average) | 4.5/5.5 | 2/2.14 | 3.5/3.83 |
| # of patients with previous splenectomy | 4 | 0 | 6 |
| patients with previous Rituximab treatments | 5 | 4 | 9 |
| Baseline AIPF (tapering counts) (*10E2/uL) (median/average) | 81.75/75.5 | 63/57.43 | 75/69 |
| Comparison Table (Responders, Tapering, Non-Responders) . | Responders (n=10) . | Tapering (n=10) . | Non-Responders (n=12) . |
|---|---|---|---|
| Age (years) (median/average) | 29/37.4 | 11/25.70 | 21/30.58 |
| Gender (m/f) | 3/7 | 6/4 | 5/7 |
| Duration ITP (years) (median/average) | 8.75/11.95 | 5.5/17.45 | 7/8.25 |
| Duration of TPO-RA Therapy (years) (median/average) | 5/4.2 | 5.5/7.45 | 2.5/3.38 |
| # of prior ITP treatments (median/average) | 4.5/5.5 | 2/2.14 | 3.5/3.83 |
| # of patients with previous splenectomy | 4 | 0 | 6 |
| patients with previous Rituximab treatments | 5 | 4 | 9 |
| Baseline AIPF (tapering counts) (*10E2/uL) (median/average) | 81.75/75.5 | 63/57.43 | 75/69 |
Bussel:Immunomedics: Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; BiologicTx: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Momenta: Membership on an entity's Board of Directors or advisory committees; Symphogen: Membership on an entity's Board of Directors or advisory committees; Protalex: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal