Key Points
Circulating CD49d+VEGFR1highCXCR4high neutrophils that stimulate angiogenesis at sites of hypoxia were identified in mice and humans.
This subset was recruited to tissue by VEGF-A in a VEGFR1- and VEGFR2-dependent manner, and anti-CD49d therapy inhibited their extravasation.
Abstract
Vascular endothelial growth factor A (VEGF-A) is upregulated during hypoxia and is the major regulator of angiogenesis. VEGF-A expression has also been found to recruit myeloid cells to ischemic tissues where they contribute to angiogenesis. This study investigates the mechanisms underlying neutrophil recruitment to VEGF-A as well as the characteristics of these neutrophils. A previously undefined circulating subset of neutrophils shown to be CD49d+VEGFR1highCXCR4high was identified in mice and humans. By using chimeric mice with impaired VEGF receptor 1 (VEGFR1) or VEGFR2 signaling (Flt-1tk−/−, tsad−/−), we found that parallel activation of VEGFR1 on neutrophils and VEGFR2 on endothelial cells was required for VEGF-A-induced recruitment of circulating neutrophils to tissue. Intravital microscopy of mouse microcirculation revealed that neutrophil recruitment by VEGF-A versus by the chemokine macrophage inflammatory protein 2 (MIP-2 [CXCL2]) involved the same steps of the recruitment cascade but that an additional neutrophil integrin (eg, VLA-4 [CD49d/CD29]) played a crucial role in neutrophil crawling and emigration to VEGF-A. Isolated CD49d+ neutrophils featured increased chemokinesis but not chemotaxis compared with CD49d– neutrophils in the presence of VEGF-A. Finally, by targeting the integrin α4 subunit (CD49d) in a transplantation-based angiogenesis model that used avascular pancreatic islets transplanted to striated muscle, we demonstrated that inhibiting the recruitment of circulating proangiogenic neutrophils to hypoxic tissue impairs vessel neoformation. Thus, angiogenesis can be modulated by targeting cell-surface receptors specifically involved in VEGF-A-dependent recruitment of proangiogenic neutrophils without compromising recruitment of the neutrophil population involved in the immune response to pathogens.
Introduction
Hypoxia is closely linked to inflammation, and numerous bone marrow–derived myeloid cells are detected at sites of tissue ischemia.1 The importance of these cells, encompassing macrophages, monocytes, and neutrophils, in restoring blood flow to hypoxic tissue has been recognized.2-5 Macrophages, monocytes, and neutrophils can stimulate angiogenesis by delivering proangiogenic growth factors (eg, vascular endothelial growth factor A [VEGF-A] and VEGF-C) as well as by the release of proteinases (eg, matrix metalloproteinase 9 [MMP-9]) that digest extracellular matrix and thereby release matrix-bound growth factors and allow for vessel sprouting.6-8 Neutrophils represent the major leukocyte subset in blood. Their recruitment from circulation to inflammatory foci has been extensively studied and is the result of sequential steps of the recruitment cascade.9,10 The mechanisms underlying homing of neutrophils to hypoxic sites remain unknown.
Delineating the underlying mechanisms of trafficking of proangiogenic leukocytes to sites of hypoxia is essential for therapeutic regulation of local angiogenesis. A direct role for VEGF-A in leukocyte trafficking has not yet been defined, but indirect evidence is accumulating. By using a transgenic system, local VEGF-A overproduction was demonstrated to induce recruitment of myeloid cells (monocytes) to a targeted organ.6,11 In addition, we have previously demonstrated that VEGF-A production by intramuscularly transplanted pancreatic islets is crucial for recruitment of a neutrophil subset that delivers high concentrations of MMP-9 and thereby induces revascularization of the hypoxic grafts.8
VEGF-A expression is upregulated by hypoxia and transmits its proangiogenic effects by binding receptors on endothelial cells. Endothelial activation by VEGF-A predominantly occurs after ligation of VEGF receptor 2 (VEGFR2; also known as kinase insert domain receptor [KDR] or fetal liver kinase 1 [Flk1]), but lower levels of VEGF receptor 1 (VEGFR1; also known as fms-like tyrosine kinase-1 [Flt-1]) with uncertain signaling properties are also detected on endothelium. Low levels of VEGFR2 expression have also been reported on certain nonendothelial cells, including neuronal cells, osteoblasts, pancreatic duct cells, retinal progenitor cells, and megakaryocytes.12 In addition, VEGFR1 has been found on monocytes, macrophages, and pericytes,13,14 but the biological role of the receptor expression on these cells is not completely understood.
This study aimed to uncover the mechanisms underlying VEGF-A-stimulated recruitment of neutrophils and to further characterize these cells. Here we show that neutrophil recruitment to VEGF-A was dependent on parallel activation of VEGFR2 on endothelium and VEGFR1 on neutrophils. Neutrophil integrin very late antigen-4 (VLA-4; an integrin composed of CD49d [α 4] and CD29 [β 1]) was used during VEGF-A-induced recruitment, and CD49d neutralization caused the vast majority of crawling neutrophils to detach from the vessel wall. Consequently, anti-CD49d therapy completely inhibited neutrophil emigration in VEGF-A-activated muscle, whereas neutrophil recruitment to macrophage inflammatory protein 2 (MIP-2 [CXCL2])-induced inflammation was not impaired. Ultimately, inhibition of CD49d in an in vivo model of angiogenesis resulted in a decreased number of neutrophils at the hypoxic site as well as impaired vessel neoformation. Taken together, our data unravel the identity of a circulating neutrophil subset with proangiogenic properties that home to ischemic tissue in a VEGFR1/VEGFR2- and VLA-4-dependent manner and that their presence is required for efficient angiogenesis. In mouse and human circulation, the distinct CD49d+ neutrophil population specifically expressed VEGFR1.
Methods
An extended “Methods” section can be found in the supplemental Data, available at the Blood Web site.
Animals
Animals used in this study were wild-type (WT) C57BL/6 mice (Taconic Bomholt, Ry, Denmark), CD45.1+ mice (The Jackson Laboratory), CX3CR1GFP/GFP mice (The Jackson Laboratory), tsad−/− mice15 (kindly provided by Professor Jeffrey A. Bluestone, University of California at San Francisco, San Francisco, CA), and Flt-1 tk−/− mice16 (kindly provided by Professor Yihai Cao, Karolinska Institute, Stockholm, Sweden), all on C57BL/6 background and all weighing 20 to 35 g. All procedures were approved by the Regional Animal Ethics Committee in Uppsala, Sweden.
Exposure of the cremaster muscle
The left cremaster muscle of the anesthetized mouse was exposed and mounted for intravital microscopic observation as previously described by others and us.17,18 The exposed muscle was continuously superperfused with a bicarbonate-buffered saline solution. A catheter was inserted in the left femoral artery allowing retrograde close intra-arterial (c.i.a.) infusion to the muscle microcirculation.
Induction of leukocyte recruitment
Recombinant murine VEGF-A165 (0.2-20 nM; Peprotech EC Ltd, Rocky Hill, NJ) or the chemokine MIP-2 (CXCL2) (0.5 nM; R&D Systems, Minneapolis, MN) was added to the bicarbonate buffer to induce leukocyte recruitment. To investigate the role of VEGFRs, animals were pretreated (c.i.a. infusion 30 minutes before start of experiment) with neutralizing antibodies against VEGFR1 (40 mg/kg; [Flt-1, clone MF1]; Eli Lilly and Company), VEGFR2 (40 mg/kg; [Flk1, clone DC101]; Eli Lilly and Company), or isotype control antibody (rat immunoglobulin G1 kappa [IgG1κ]).
Intravital video microscopy
Five-minute long periods were recorded in real time format (Leica DM5000B, Wetzlar, Germany) immediately before (0 minutes, basal) and at 30, 60, and 90 minutes after addition of the stimulus to the superperfusate. Rolling, adhesion, and transmigration were quantified by using video playback analysis (Improvision Volocity Acquisition Software, Coventy, UK). Evaluation of intraluminal crawling was performed in separate experiments where images were acquired in time lapse format (1 frame/10 sec) on the selected section of venule between 30 to 90 minutes after addition of the stimulus to the superperfusate.
To enable distinction between leukocyte subtypes, monocytes were distinguished from neutrophils by using CX3CR1GFP/GFP mice or by immunolabeling with fluorescently tagged CD115 monoclonal antibody (mAb; clone AFS98; eBioscience, San Diego, CA) conjugated to Alexa Fluor Dye 488 (Invitrogen, Life Technologies, Eugene, OR), and neutrophils were immunostained in vivo by c.i.a. administration of Ly6G mAb (clone 1A8; BD Biosciences, Heidelberg, Germany) conjugated to Alexa Fluor Dye 555 (Invitrogen, Life Technologies).
For assessing the role of neutrophil surface integrins lymphocyte-associated antigen 1 (LFA-1), macrophage antigen 1 (Mac-1), or VLA-4 in in vivo leukocyte recruitment, 30 μg of neutralizing mAbs was administered (c.i.a. infusion) 30 minutes before basal measurements by intravital microscopy. Anti-mouse CD11a (clone M17/4), anti-mouse CD11b (clone M1/70), or anti-mouse CD49d (clone R1-2), respectively, was used. All antibodies were from eBioscience. For controls, isotype control antibodies (rat IgG2aκ and rat IgG2bκ) were administered.
Bone marrow transplantation
Isolated bone marrow cells (1.5 × 106) were transplanted into congenic recipients by retro-orbital injection. Recipients were irradiated with a split dose of 10 Gy in a 137Cs irradiator (MDS Nordion, Ottawa, ON, Canada). The following experimental groups were used: WT CD45.2+ to WT CD45.1+, Flt-1 tk−/− CD45.2+ to WT CD45.1+, and tsad−/− CD45.2+ to WT CD45.1+. Peripheral blood chimerism was determined 5 weeks after transplantation. In vivo experiments regarding neutrophil recruitment by VEGF-A were performed 16 weeks after the bone marrow transplantation.
Isolation of human peripheral blood neutrophils
Human neutrophils were purified as previously described.19 Briefly, blood from healthy volunteers was collected, and neutrophils were isolated by using erythrocyte dextran sedimentation followed by 2 rounds of hypotonic lysis (4°C). Neutrophils were isolated from the resulting cell suspension by using Ficoll (GE Healthcare Life Sciences, Uppsala, Sweden) density gradient separation.
Isolation of mouse peripheral blood neutrophils
Mouse neutrophils were purified from blood collected by cardiac puncture as previously described.20 Leukocytes were isolated by using erythrocyte dextran sedimentation followed by 2 rounds of hypotonic lysis (4°C). Neutrophils were isolated from the resulting cell suspension by using a Percoll (GE Healthcare Life Sciences) density gradient separation.
mRNA isolation and real-time reverse-transcriptase polymerase chain reaction
Messenger RNA (mRNA) was isolated from purified mouse neutrophils by using an RNeasy Mini kit (QIAGEN, Solna, Sweden). All of the polymerase chain reactions were run on a LightCycler (Roche Diagnostics, Basel, Switzerland). The cycle threshold (CT) values were estimated with LightCycler Software, v4.1, and transcript levels were normalized by subtracting the corresponding β-actin values (ΔCt). Relative expression was determined as values normalized against endothelial VEGFR2 by the equation 2–ΔCt–ECVEGFR2ΔCt.
ERK activation
Extracellular signal-regulated kinase (ERK) activation was assayed by using standard western blotting techniques. Briefly, 1 × 106 human neutrophils were stimulated with 2 nM recombinant human VEGF-A165 or 5 nM recombinant human VEGF-B167 (both from Peprotech) after a 3-hour period of serum deprivation.
Flow cytometry
All flow cytometric analyses were carried out on LSR II or FACSCalibur instruments (both from BD Bioscience) and data were processed by using FlowJo software (TreeStar, Ashland, OR) or by using Flowing Software (Turku Bioimaging, University of Turku, Turku, Finland). See supplemental Data for a full list of antibodies.
Neutrophil chemotaxis in response to gradients of interleukin-8 (IL-8) and VEGF-A
Populations of human CD49d+ and CD49d– neutrophils were sorted on a FACSAria II sorter (BD Biosciences). Chemotaxis of CD49d+ and CD49d– neutrophils was assessed by using the Cell Director 2D chemotaxis assay (Gradientech, Uppsala, Sweden).
Pancreatic islet isolation and transplantation
Mouse islets were isolated as described earlier.21 Briefly, islets were separated from exocrine tissue by collagenase digestion and density gradient centrifugation (Histopaque-1077, Sigma-Aldrich). Purified islets were then hand-picked and were maintained as free-floating cells in islet culture medium. In some cases, islets were fluorescently labeled with the Celltracker Blue CMAC intracellular probe (Invitrogen, Life Technologies) immediately before transplantation through a butterfly needle to the cremaster muscle or to the external abdominal oblique muscle of anesthetized mice.
Intravital confocal imaging of neutrophil recruitment to hypoxic pancreatic islets transplanted to muscle
A line-scanning confocal microscope (Zeiss LSM 5 Live, with a piezo motor-controlled W Plan Apo 40/1.0 objective with 0.5-2.0× optical zoom, and Zeiss Zen 2009 software) was used to visualize islet grafts, vasculature, and neutrophils in the cremaster muscle of mice that had received transplantation and had been treated for 5 days with neutralizing mAbs toward CD49d (30 μg intravenously injected via tail vein per mouse and per day, 24 hours prior to transplantation and every 24 hours until the day of the experiment [ie, 4 days after transplantation]). Transplanted mice treated with isotype control (IgG2bκ) were used as controls.
Statistics
All data are presented as mean ± standard error of the mean. Unpaired Student t tests or analysis of variance (one-way or two-way) with Bonferroni correction were used for comparison between 2 or more groups. For verifying single variable changes with time and within 1 group, paired Student t tests or analysis of variance (repeated measures) with Bonferroni correction were used for comparison between 2 or more time points. Statistical significance was considered for probability values (P) less than .05.
Results
VEGF-A induces rapid and robust recruitment of neutrophils to tissue
Real-time intravital video microscopy of the mouse cremaster muscle demonstrated significantly increased numbers of adherent and emigrated leukocytes after 30 minutes of superperfusion with 2 or 20 nM of VEGF-A (Figure 1A-B). Lower concentrations of VEGF-A in the superperfusion buffer (0.2 and 0.4 nM) did not induce leukocyte recruitment (Figure 1A-B). The VEGF-A-recruited leukocytes were demonstrated to be neutrophils and not monocytes (Figure 1C-D) by using in vivo fluorescent labeling of the different populations.
VEGF-A rapidly induces recruitment of circulating neutrophils to tissue in a VEGFR1- and VEGFR2-dependent manner. VEGF-A-induced leukocyte (A) intravascular adhesion and (B) emigration occur within 30 minutes after onset of VEGF-A superperfusion of the cremaster muscle (control [0 nM], n = 4; VEGF-A 0.2 nM, n = 4; VEGF-A 0.4 nM, n = 4; VEGF-A 2 nM, n = 6; and VEGF-A 20 nM, n = 6). #P < .05 compared with basal values within the same group; *P < .05 compared with control group at the same time point. Leukocyte subsets were differentiated by using CX3CR1GFP/GFP mice and fluorescently tagged anti-Ly6G mAb (clone 1A8). (C) One frame from time 30 minutes in a video recording is shown. Scale bar = 30 μm. Postcapillary venule outlined with dashed lines. (D) The leukocytes recruited after 90 minutes of VEGF-A (2 nM) superperfusion were neutrophils (Ly6G+) and not monocytes (CX3CR1+; control, n = 3; VEGF-A, n = 6). The VEGF-A-induced neutrophil recruitment to muscle was completely inhibited by neutralizing antibodies directed toward either VEGFR1 (clone MF1) or VEGFR2 (clone DC101). (E) Adherent cells and (F) emigrated cells (n = 5 per treatment group). *P < .05 compared with control group at the same time point. (G) Mouse peripheral blood neutrophils expressed levels of VEGFR1 transcripts similar to those in endothelial cells. Relative expression of mRNA presented in arbitrary units (A.U.). Semiquantitative reverse-transcription polymerase chain reaction (n = 11 mice) from 3 independent trials. (H) VEGFR1 and VEGFR2 protein expressions were confirmed on circulating human neutrophils (n = 8) by flow cytometry (isotype in black). (I) Direct stimulation of human neutrophils by either VEGF-A (2 nM) or VEGF-B (5 nM) induced signal transduction via VEGFR1 (phosphorylation of ERK [pERK] compared to total Erk [tErk]; western blot from 4 independent trials).
VEGF-A rapidly induces recruitment of circulating neutrophils to tissue in a VEGFR1- and VEGFR2-dependent manner. VEGF-A-induced leukocyte (A) intravascular adhesion and (B) emigration occur within 30 minutes after onset of VEGF-A superperfusion of the cremaster muscle (control [0 nM], n = 4; VEGF-A 0.2 nM, n = 4; VEGF-A 0.4 nM, n = 4; VEGF-A 2 nM, n = 6; and VEGF-A 20 nM, n = 6). #P < .05 compared with basal values within the same group; *P < .05 compared with control group at the same time point. Leukocyte subsets were differentiated by using CX3CR1GFP/GFP mice and fluorescently tagged anti-Ly6G mAb (clone 1A8). (C) One frame from time 30 minutes in a video recording is shown. Scale bar = 30 μm. Postcapillary venule outlined with dashed lines. (D) The leukocytes recruited after 90 minutes of VEGF-A (2 nM) superperfusion were neutrophils (Ly6G+) and not monocytes (CX3CR1+; control, n = 3; VEGF-A, n = 6). The VEGF-A-induced neutrophil recruitment to muscle was completely inhibited by neutralizing antibodies directed toward either VEGFR1 (clone MF1) or VEGFR2 (clone DC101). (E) Adherent cells and (F) emigrated cells (n = 5 per treatment group). *P < .05 compared with control group at the same time point. (G) Mouse peripheral blood neutrophils expressed levels of VEGFR1 transcripts similar to those in endothelial cells. Relative expression of mRNA presented in arbitrary units (A.U.). Semiquantitative reverse-transcription polymerase chain reaction (n = 11 mice) from 3 independent trials. (H) VEGFR1 and VEGFR2 protein expressions were confirmed on circulating human neutrophils (n = 8) by flow cytometry (isotype in black). (I) Direct stimulation of human neutrophils by either VEGF-A (2 nM) or VEGF-B (5 nM) induced signal transduction via VEGFR1 (phosphorylation of ERK [pERK] compared to total Erk [tErk]; western blot from 4 independent trials).
In vivo neutrophil recruitment by VEGF-A depends on activation of VEGFR1 and VEGFR2
To investigate the role of the two VEGF-A-binding receptors VEGFR1 (Flt-1) and VEGFR2 (Flk-1/KDR) in VEGF-A-induced neutrophil recruitment, neutralizing antibodies directed against either VEGFR1 or VEGFR2 were used (clones MF1 and DC101, respectively22 ). Blockade of VEGF-A binding to either receptor inhibited both neutrophil adhesion and emigration (Figure 1E-F) during 60 minutes of VEGF-A stimulation (2 nM), demonstrating that both these receptors participate in the in vivo recruitment of neutrophils by VEGF-A.
VEGF-A directly activates VEGFR1 signaling in neutrophils
Potential direct effects of VEGF-A on neutrophils were investigated by measuring neutrophil expression of VEGFR1 and VEGFR2 using semiquantitative reverse transcription polymerase chain reaction. Isolated mouse neutrophils and endothelial cells expressed similar levels of VEGFR1 transcripts, whereas neutrophils expressed very low levels of VEGFR2 transcripts (Figure 1G). Flow cytometry confirmed VEGFR1 protein expression on circulating neutrophils from both mouse blood (Ly6G+; anti-VEGFR1: MFI [mean fluorescence intensity], 19.0 ± 2.4; isotype control: MFI, 1.6 ± 0.1; n = 5) and human blood (CD16+; anti-VEGFR1: MFI, 35.2 ± 8.0; isotype control: MFI, 10 ± 1; n = 8; Figure 1H). No VEGFR2 expression on human neutrophils was found by using flow cytometry (Figure 1H).
To verify VEGFR1 activity in neutrophils, isolated human neutrophils were exposed to VEGF-A or to the specific VEGFR1 ligand VEGF-B23-26 for different time periods. After VEGF-A stimulation, a rapid increase in neutrophil phosphorylated ERK downstream of receptor ligation was detected (Figure 1I and supplemental Figure 1). VEGF-B also induced ERK phosphorylation but with slower kinetics than VEGF-A in accordance with previous findings (Figure 1J and supplemental Figure 127 ). By using the VEGFR2-specific ligand VEGF-E, no ERK activity in neutrophils was recorded (data not shown). These results demonstrate that VEGFR1 is expressed on neutrophils and can be activated by VEGF-A.
VEGFR1 on neutrophils and VEGFR2 on endothelium are necessary for neutrophil recruitment by VEGF-A
Intravital microscopy of the cremaster muscle of mice that lacked the tyrosine kinase domain of VEGFR1 (Flt-1 tk−/−) revealed that neutrophil recruitment by VEGF-A was completely prevented (Figure 2A-B), whereas neutrophil recruitment by the chemokine MIP-2 was preserved (Figure 2C-D). These observations are in agreement with the results using VEGFR1 neutralizing antibodies (Figure 1E-F) and demonstrate that VEGFR1 is not involved in neutrophil extravasation to MIP-2. These experiments were repeated in T-cell-specific adapter protein (TSAd)-deficient mice (tsad−/−) to determine the role of VEGFR2 for VEGF-A-dependent neutrophil recruitment. TSAd is expressed in endothelial cells and also in some immune cells,28,29 and tsad−/− mice are characterized by disrupted VEGFR2-Src kinase signaling.28,29 By using tsad−/− mice, we found that loss of VEGFR2-Src signaling compromised the capacity of VEGF-A to induce neutrophil recruitment (Figure 2A-B), consistent with our results using VEGFR2-neutralizing antibodies (Figure 1E-F). Nevertheless, MIP-2-induced neutrophil recruitment was unaffected by TSAd deficiency (Figure 2C-D).
Activation of both VEGFR1 on neutrophils and VEGFR2 on endothelium is necessary for in vivo VEGF-A-induced neutrophil recruitment. (A-B) Specific knockdown of either VEGFR1 tyrosine kinase (Flt-1 tk−/− mice) or VEGFR2-TSAd-Src signaling (tsad−/− mice) inhibited VEGF-A (2 nM)-dependent neutrophil recruitment to the cremaster muscle (n = 4-7 mice per group). The number of (C) adherent and (D) emigrated neutrophils recruited in vivo by the proinflammatory chemokine MIP-2 (CXCL2; 0.5 nM) in the cremaster muscle of both Flt-1 tk−/− and tsad−/− mice was similar to that in the cremaster muscle of WT mice (n = 5-6 mice per group). (E-F) VEGF-A-dependent neutrophil recruitment was inhibited in WT mice transplanted with Flt-1 tk−/− bone marrow whereas neutrophil recruitment to VEGF-A was unaffected in WT mice transplanted with tsad−/− bone marrow (n = 5-6 mice per group). #P, .05 compared with basal values within the same group; *P < .05 compared with other groups, as indicated.
Activation of both VEGFR1 on neutrophils and VEGFR2 on endothelium is necessary for in vivo VEGF-A-induced neutrophil recruitment. (A-B) Specific knockdown of either VEGFR1 tyrosine kinase (Flt-1 tk−/− mice) or VEGFR2-TSAd-Src signaling (tsad−/− mice) inhibited VEGF-A (2 nM)-dependent neutrophil recruitment to the cremaster muscle (n = 4-7 mice per group). The number of (C) adherent and (D) emigrated neutrophils recruited in vivo by the proinflammatory chemokine MIP-2 (CXCL2; 0.5 nM) in the cremaster muscle of both Flt-1 tk−/− and tsad−/− mice was similar to that in the cremaster muscle of WT mice (n = 5-6 mice per group). (E-F) VEGF-A-dependent neutrophil recruitment was inhibited in WT mice transplanted with Flt-1 tk−/− bone marrow whereas neutrophil recruitment to VEGF-A was unaffected in WT mice transplanted with tsad−/− bone marrow (n = 5-6 mice per group). #P, .05 compared with basal values within the same group; *P < .05 compared with other groups, as indicated.
VEGFR1 signaling on either neutrophils or endothelial cells could explain the in vivo VEGF-A recruitment data. To distinguish these two possibilities, chimeric mice were prepared by transplanting WT or Flt-1 tk−/− bone marrow to irradiated WT mice (donor cell chimerism >85% for all mice). VEGF-A superperfusion of the cremaster muscle of WT mice transplanted with WT bone marrow (WT to WT) resulted in increased adherent and emigrated neutrophils (Figure 2E-F) to levels previously observed in WT mice (Figure 1A-B). In contrast, no neutrophils were recruited in response to VEGF-A in WT mice transplanted with Flt-1 tk−/− bone marrow (Flt-1 tk−/− to WT; Figure 2E-F), demonstrating that VEGFR1 signaling on bone marrow–derived cells is indispensable for VEGF-A-induced neutrophil recruitment.
To determine whether endothelial or neutrophil expression of VEGFR2 was essential for VEGF-A-induced leukocyte recruitment, chimeric mice were constructed through transplantation of tsad−/− bone marrow into irradiated WT hosts (tsad−/− to WT; donor cell chimerism >85% for all mice). Leukocytes from tsad−/− mice adhered to and transmigrated through the venular walls of irradiated WT mice to an extent similar to that in WT leukocytes in WT mice (WT to WT; Figure 2E-F). Thus, the reduced leukocyte recruitment in tsad−/− mice in response to VEGF-A was due to loss of VEGFR2 signaling in nonhematopoietic cells.
Neutrophils engage different adhesion molecules when recruitment is mediated by VEGF-A rather than by an inflammatory chemokine
To investigate the adhesion molecules used during recruitment of neutrophils by VEGF-A, neutralizing mAbs directed toward the α unit of the 3 major neutrophil integrins (ie, anti-CD11a, anti-CD11b, and anti-CD49d for neutralization of LFA-1 [integrin αLβ2; CD11a/CD18], Mac-1 [integrin αMβ2; CD11b/CD18], and VLA-4 [integrin α4β1; CD49d/CD29], respectively) were administered via c.i.a. infusion to the cremaster muscle prior to VEGF-A stimulation. A striking reduction of adherent neutrophils (Ly6G clone 1A8-positive leukocytes) was observed in VEGF-A-stimulated mice (90 minutes of stimulation) pretreated with anti-CD11a or anti-CD11b (Figure 3A), which translated into decreased numbers of emigrated neutrophils (Figure 3B). Treatment with anti-CD49d did not significantly alter the number of adherent neutrophils in response to VEGF-A but totally inhibited emigration out of the vasculature (Figure 3A-B). When the efficacy of emigration was calculated (number of emigrated cells per number of adherent cells), it was evident that anti-CD49d effectively and significantly inhibited the emigration step compared with what was observed for the other inhibitors (isotype, 1.3 ± 0.2; anti-CD11a, 1.0 ± 0.2; anti-CD11b, 1.8 ± 0.8; anti-CD49d, 0.3 ± 0.1). In-depth analysis of the adherent cells revealed that both Mac-1 and VLA-4 were involved in intravascular crawling of neutrophils (Figure 3C) but only anti-CD49d treatment caused the majority of crawling cells to detach (Figure 3D). Consequently, emigration to the tissue was completely inhibited by anti-CD49d in VEGF-A-activated muscle. In contrast, neutralization of VLA-4 did not impair neutrophil recruitment to MIP-2-induced inflammation (supplemental Figure 2A-D), which was demonstrated to involve LFA-1 and Mac-1 only, as previously shown.30
Neutrophils use VLA-4 integrin when recruited by VEGF-A. The role of the 3 major leukocyte integrins (LFA-1, Mac-1, and VLA-4) in neutrophil recruitment by VEGF-A was accessed by intravital fluorescence video microscopy of the cremaster muscle of mice pretreated with neutralizing mAbs directed to CD11a, CD11b, or CD49d. Neutrophils were immunostained in vivo with Ly6G (clone 1A8) mAb conjugated to Alexa Fluor Dye 555 prior to the experiment. The number of (A) adherent and (B) emigrated neutrophils, as well as the fraction of intravascular (C) crawling and (D) detaching neutrophils upon 90 min of stimulation with VEGF-A (n = 4-5 mice per treatment). *P < .05 compared with other groups, as indicated.
Neutrophils use VLA-4 integrin when recruited by VEGF-A. The role of the 3 major leukocyte integrins (LFA-1, Mac-1, and VLA-4) in neutrophil recruitment by VEGF-A was accessed by intravital fluorescence video microscopy of the cremaster muscle of mice pretreated with neutralizing mAbs directed to CD11a, CD11b, or CD49d. Neutrophils were immunostained in vivo with Ly6G (clone 1A8) mAb conjugated to Alexa Fluor Dye 555 prior to the experiment. The number of (A) adherent and (B) emigrated neutrophils, as well as the fraction of intravascular (C) crawling and (D) detaching neutrophils upon 90 min of stimulation with VEGF-A (n = 4-5 mice per treatment). *P < .05 compared with other groups, as indicated.
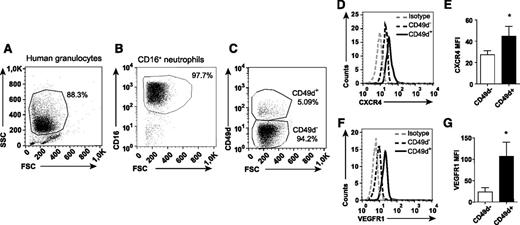
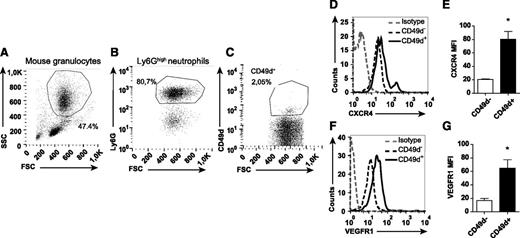
Identification of a distinct population of circulating CD49d+VEGFR1highCXCR4high neutrophils
The dramatic inhibiting effect of anti-CD49d treatment on VEGF-A-induced neutrophil transmigration indicates that circulating neutrophils express this integrin. By using flow cytometry, a subpopulation of circulating neutrophils was indeed found to be CD49d+ (human: 3.2% ± 0.5%, n = 12, Figure 4 A-C; mouse: 2.8% ± 0.6%, n = 16, Figure 5A-C). CD49d+ human and mouse neutrophils expressed significantly higher CXCR4 levels compared with CD49d– neutrophils (Figures 4D-E and 5D-E). Furthermore, the CD49d+ neutrophil population also expressed higher levels of VEGFR1 (Figures 4F-G and 5F-G). Consequently, we identified a circulating VEGF-A-responsive neutrophil subpopulation coexpressing CD49d, VEGFR1, and CXCR4 (supplemental Figure 3).
The CD49d+ population of human neutrophils expresses uniquely high levels of CXCR4 and VEGFR1. Representative flow cytometry plots of human neutrophils isolated from blood, (A) gated in forward-scattered light (FSC)/side-scattered light (SSC) and (B) on CD16 expression, and (C) labeled with mAb toward CD49d. (D-G) Representative histograms and quantified mean fluorescence intensities (MFIs) of the CD49d+ and CD49d– neutrophils after staining with CXCR4 and VEGFR1 mAbs (n = 8-12 healthy human individuals). *P < .05.
The CD49d+ population of human neutrophils expresses uniquely high levels of CXCR4 and VEGFR1. Representative flow cytometry plots of human neutrophils isolated from blood, (A) gated in forward-scattered light (FSC)/side-scattered light (SSC) and (B) on CD16 expression, and (C) labeled with mAb toward CD49d. (D-G) Representative histograms and quantified mean fluorescence intensities (MFIs) of the CD49d+ and CD49d– neutrophils after staining with CXCR4 and VEGFR1 mAbs (n = 8-12 healthy human individuals). *P < .05.
Mouse neutrophils expressing CD49d show high levels of CXCR4 and VEGFR1. Representative flow cytometry plots of mouse neutrophils isolated from blood, (A) gated in FSC/SSC and on (B) Ly6G expression, and (C) labeled with mAb toward CD49d. (D-G) Representative histograms and quantified MFIs of the CD49d+ and CD49d– neutrophils after staining with CXCR4 and VEGFR1 mAbs (n = 8-16 mice). *P < .05.
Mouse neutrophils expressing CD49d show high levels of CXCR4 and VEGFR1. Representative flow cytometry plots of mouse neutrophils isolated from blood, (A) gated in FSC/SSC and on (B) Ly6G expression, and (C) labeled with mAb toward CD49d. (D-G) Representative histograms and quantified MFIs of the CD49d+ and CD49d– neutrophils after staining with CXCR4 and VEGFR1 mAbs (n = 8-16 mice). *P < .05.
Human eosinophils can also express CD49d as well as VEGFR1.31 However, the CD16+ sorted cells did not express the eosinophil-specific marker Siglec-8, confirming that they belong to the neutrophilic lineage (supplemental Figure 4A-B). Moreover, the mouse granulocytes investigated were demonstrated to be neutrophils and not eosinophils (Ly6G+CCR3–; supplemental Figure 4C-D32 ).
Taken together, these results indicate that CD49d+VEGFR1highCXCR4high neutrophils constitute a specific circulating neutrophil subtype in both mice and humans.
VEGF-A induces chemokinesis in human CD49d+ neutrophils
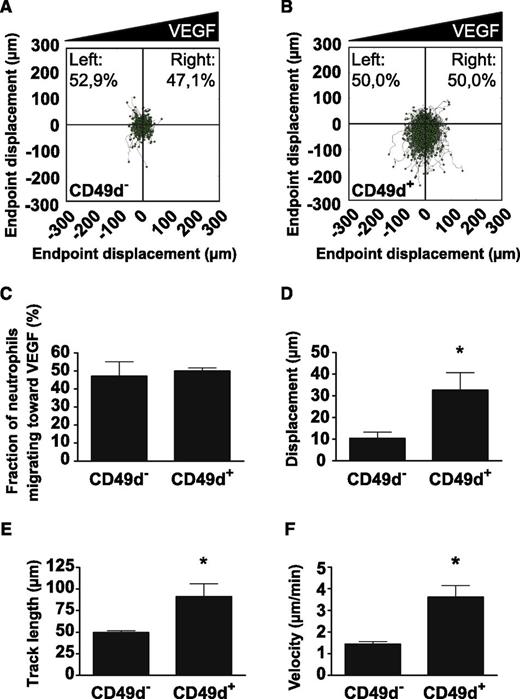
VEGF-A is a potent inducer of endothelial cell chemotaxis.33 We investigated whether VEGF-A induced neutrophil chemotaxis by using a microfluidic device after flow-sorting into CD49d+ and CD49d– populations. As a positive control, a gradient of IL-8 ranging between 0 and 50 ng/mL over a distance of ∼500 µm was shown to potently induce chemotaxis of isolated human neutrophils (supplemental Figure 5A). Interestingly, a gradient of VEGF-A (0-20 ng/mL over ∼500 µm) significantly increased chemokinesis (Figure 6A-B, D-F and supplemental Figure 5B-G) but not chemotaxis (Figure 6A-C) in the CD49d+ cell population whereas CD49d– cells did not respond to the VEGF-A stimulus. More specifically, the CD49d+ neutrophils were shown to cover longer distances (Figure 6D-E and supplemental Figure 5F-G) and to move more than twice as fast in response to VEGF-A (Figure 6F) compared with CD49d– neutrophils.
VEGF-A increases chemokinesis of CD49d+ human neutrophils. Polar plots displaying the trajectories of (A) CD49d– or (B) CD49d+ human neutrophils migrating in stable gradients of VEGF-A (from 4 independent trials). Gradients were formed with high concentration to the right side of the plots. (C) Neutrophils did not show chemotaxis in response to the VEGF-A gradient. Analysis of neutrophil migration by measuring (D) the average displacement, (E) the average track length, and (F) the average velocity per migrating neutrophil show that CD49d+ neutrophils display enhanced chemokinesis in the presence of VEGF-A. *P < .05.
VEGF-A increases chemokinesis of CD49d+ human neutrophils. Polar plots displaying the trajectories of (A) CD49d– or (B) CD49d+ human neutrophils migrating in stable gradients of VEGF-A (from 4 independent trials). Gradients were formed with high concentration to the right side of the plots. (C) Neutrophils did not show chemotaxis in response to the VEGF-A gradient. Analysis of neutrophil migration by measuring (D) the average displacement, (E) the average track length, and (F) the average velocity per migrating neutrophil show that CD49d+ neutrophils display enhanced chemokinesis in the presence of VEGF-A. *P < .05.
Disturbed revascularization of intramuscularly transplanted pancreatic islets in recipients receiving long-term CD49d neutralization
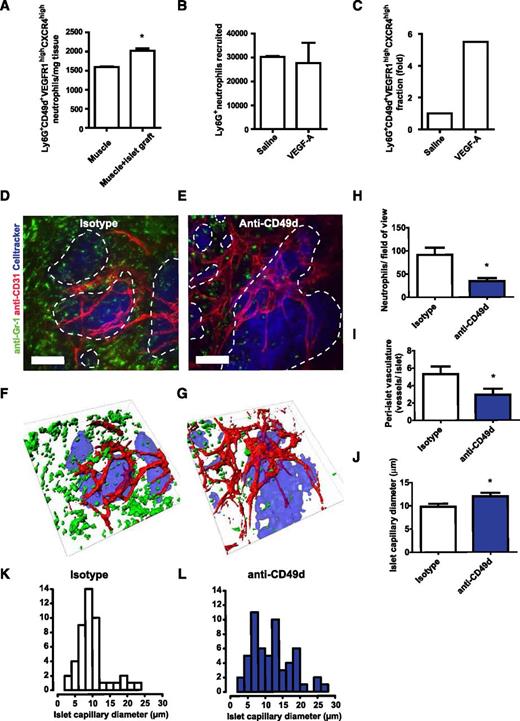
The ultimate contribution of the circulating neutrophil subtype CD49d+VEGFR1highCXCR4high to angiogenesis was investigated by using our previously established in vivo model of VEGF-A-driven islet graft revascularization.4,7 When isolated pancreatic islets were syngeneically transplanted into striated muscle of mice, a 10-fold increase of neutrophils (Ly6G+) was observed 24 hours after transplantation (results not shown). The number of Ly6G+CD49d+VEGFR1highCXCR4high neutrophils were also significantly increased compared with normal muscle tissue (Figure 7A). This population of neutrophils was moreover found to be responsive to VEGF-A in a peritoneal recruitment model. Even though similar numbers of neutrophils were found in the peritoneum of saline- or VEGF-A-treated mice (i.p.), the Ly6G+CD49d+VEGFR1highCXCR4high neutrophil subset was increased nearly sixfold in the VEGF-A group (Figure 7B-C). To examine the possibility of intervening with the recruitment of proangiogenic neutrophils in this islet graft revascularization model, transplanted mice were treated daily (intravenously starting 24 hours before transplantation) with anti-CD49d-neutralizing antibodies during the islet engraftment period. CD49d inhibition did not affect blood leukocyte counts or the circulating neutrophil fraction, neither leukocyte-endothelial cell interactions under basal conditions in postcapillary venules (supplemental Figure 6A-E).
CD49d is important for neutrophil recruitment to and revascularization of avascular islets. (A) Ly6G+CD49d+VEGFR1highCXCR4high neutrophils are enriched in muscles transplanted with avascular pancreatic islets. (B) Numbers of neutrophils (Ly6G+) in the peritoneal cavity of mice 5 hours post i.p. injection with saline (n = 2) or VEGF-A (n = 4) and (C) the proportion of the proangiogenic Ly6G+CD49d+VEGFR1highCXCR4high subpopulation. (D-E) Representative confocal Z-projection images of transplanted pancreatic islets (blue, dashed lines) in the cremaster muscle of mice treated with isotype control or anti-CD49d antibodies during islet engraftment, and (F-G) corresponding surface renderings. Images acquired by in vivo confocal microscopy 4 days after transplantation (n = 6 mice per treatment group; 18 islets were analyzed for the isotype control and 21 islets were analyzed for the anti-CD49d-treated group). Scale bars = 50 μm. (H) In mice in which CD49d was inhibited, less than half the amounts of recruited neutrophils (green) were found surrounding the graft compared with isotype-treated animals. (I) Blood vessels (red) surrounding the islets were quantified and (J) islet capillary diameter was measured. (L) The capillary diameters in anti-CD49d treated animals were more heterogeneous than (K) those in animals treated with isotype control antibodies. *P < .05.
CD49d is important for neutrophil recruitment to and revascularization of avascular islets. (A) Ly6G+CD49d+VEGFR1highCXCR4high neutrophils are enriched in muscles transplanted with avascular pancreatic islets. (B) Numbers of neutrophils (Ly6G+) in the peritoneal cavity of mice 5 hours post i.p. injection with saline (n = 2) or VEGF-A (n = 4) and (C) the proportion of the proangiogenic Ly6G+CD49d+VEGFR1highCXCR4high subpopulation. (D-E) Representative confocal Z-projection images of transplanted pancreatic islets (blue, dashed lines) in the cremaster muscle of mice treated with isotype control or anti-CD49d antibodies during islet engraftment, and (F-G) corresponding surface renderings. Images acquired by in vivo confocal microscopy 4 days after transplantation (n = 6 mice per treatment group; 18 islets were analyzed for the isotype control and 21 islets were analyzed for the anti-CD49d-treated group). Scale bars = 50 μm. (H) In mice in which CD49d was inhibited, less than half the amounts of recruited neutrophils (green) were found surrounding the graft compared with isotype-treated animals. (I) Blood vessels (red) surrounding the islets were quantified and (J) islet capillary diameter was measured. (L) The capillary diameters in anti-CD49d treated animals were more heterogeneous than (K) those in animals treated with isotype control antibodies. *P < .05.
Grafts were examined by in vivo confocal microscopy 4 days after transplantation (Figure 7D-G). A 62% decrease in the number of neutrophils (Ly6G+) at the site of islet engraftment was observed upon CD49d neutralization compared with mice receiving isotype (Figure 7H), but no effect on the recruitment of monocytes and macrophages (CX3CR1GFP cells) was noticed (supplemental Figure 7A-C). The Ly6G+ cells that were responsive to VEGF-A stimulus were also found to be exclusively neutrophils, and CX3CR1+ and/or CD115+ monocytes in these settings were found to be Ly6Glow and Gr-1low (supplemental Figure 8).
During the first few days following islet transplantation, the angiogenic activity is most intense in the area surrounding the grafts rather than inside the grafts.4 Neovascularization was therefore quantified as the number of CD31+ vessels surrounding each islet graft within 30 µm of the islet perimeter. Preventing the recruitment of CD49d+ neutrophils by anti-CD49d treatment decreased the numbers of newly formed islet-juxtaposed vessels (Figure 7I). In addition, the newly formed vasculature often formed larger vascular plexa rather than distinct capillaries (Figure 7J-L), indicating delayed pruning of vessels. Thus, this suggests that neutrophils with proangiogenic properties are recruited to the hypoxic islets in a CD49d-dependent manner.
Human CD49d+ neutrophils exhibit proangiogenic activity
The release of proteases such as MMP-9 is considered to be one of the major mechanisms that neutrophils use to stimulate blood vessel growth.5 To examine potential proangiogenic effects of the human CD49d+ neutrophil subset, isolated human blood neutrophils were flow-sorted into Siglec8−CD16+CD49d– and Siglec8−CD16+CD49d+ populations and incubated separately in highly quenched fluorescein-labeled gels for 30 minutes. Upon proteolytic digestion of the gels, green fluorescence is emitted and can be therefore used to measure protease release (activity) from neutrophils. Gels containing CD49d+ neutrophils emitted significantly more green fluorescence than CD49d– neutrophils (supplemental Figure 9). These results suggest that the circulating human CD49d+ neutrophil population holds the potential of being a bona fide proangiogenic subset.
Discussion
In this study, we identified the presence of a circulating CD49d+ neutrophil population that expresses VEGFR1 and high levels of CXCR4 in both humans and mice (CD49d+VEGFR1highCXCR4high neutrophils). Furthermore, in a functional in vivo assay, specific means of inhibiting the recruitment of these neutrophils were uncovered because they depend on VLA-4 integrin and VEGFR1 during VEGF-A-induced recruitment to tissue, implicating these cells as a distinct subpopulation of circulating neutrophils with proangiogenic properties. This study defines a new subset of neutrophils and means of directly targeting recruitment of this cell population to sites of hypoxia.
Myeloid cells are abundant at sites of hypoxia where they contribute to angiogenesis.2-4,34,35 VEGF-A is induced by local hypoxia14,36 and is well known for its potent angiogenic effects on endothelium. Much less is known about the effects of VEGF-A on myeloid cell trafficking. VEGF-A exerts its functions through 2 receptor tyrosine kinases, VEGFR1 and VEGFR2, expressed on endothelial cells but also to various extents on pericytes, monocytes, and macrophages.12-14 This study demonstrates the presence of functional VEGFR1 on both human and mouse neutrophils. VEGF-A did not recruit neutrophils to muscle in (irradiated) WT recipient mice transplanted with Flt-1 tk−/− bone marrow, demonstrating the importance of VEGFR1 on bone marrow–derived cells. Direct actions of VEGF-A on neutrophil VEGFR1 can explain our observation of rapid onset of recruitment in response to VEGF-A. Intriguingly, VEGFR2 signaling was also found to be crucial for VEGF-A-dependent recruitment of neutrophils using VEGFR2-neutralizing antibodies and tsad−/− mice. TSAd is a VEGFR2 signal adapter molecule that activates the tyrosine-protein kinase c-Src and is crucial for the well-established effects of VEGF-A on increasing vascular permeability.29,37 This study demonstrates that VEGFR2-TSAd-Src signaling essential for neutrophil transmigration was confined to VEGFR2 expression on non-bone marrow–derived cells (presumably predominantly endothelial cells). In addition to our findings, upregulation of endothelial adhesion molecules involved in leukocyte recruitment has been demonstrated in vitro following VEGF-A exposure38-40 and following intradermal injections.41 Furthermore, VEGFR2 inhibition in vitro prevents VEGF-A-induced upregulation of mRNA levels of intercellular adhesion molecule-1 (ICAM-1 [CD54]), vascular cell adhesion molecule-1 (VCAM-1 [CD106]), and E-selectin on human umbilical vein endothelial cells.42 VEGF-A can also induce expression of certain chemokines (eg, stromal cell–derived factor-1 [SDF-1 [CXCL12]]43 ) with leukocyte recruiting abilities.44 The chemokine CXCL12 is released by endothelium45 and is mainly associated with retention of myeloid cells in the bone marrow niche. However, when CXCL12 was transgenically overexpressed, accumulation of CXCR4+ myeloid cells was observed in tissue close to blood vessels6 where they could participate in local events such as angiogenesis.
The VEGF-A-induced neutrophil recruitment was dependent on VLA-4 (integrin α4β1 [CD49d/CD29]), and long-term inhibition of this α4 integrin significantly reduced the population of neutrophils at sites of hypoxia as well as the amount of newly formed blood vessels. Our data indicate that VLA-4 is important for maintaining adhesion and preventing detachment during intravascular crawling, but the downstream mechanisms are still unknown. This finding contrasts to what is reported for neutrophil recruitment to an inflammatory stimulus, which completely depends on LFA-1 (integrin αLβ2 [CD11a/CD18]) and Mac-1 (integrin αMβ2 [CD11b/CD18]).8,9,30 In murine models however, myeloid cell trafficking into tumors has been reported to rely on VLA-4 after activation by PI3-kinase p110γ.46 Only a small population of circulating neutrophils expressed CD49d in this study, which is in accordance with the prevailing view. Neutrophils express high levels of this integrin during early maturation stages in bone marrow after which CD49d expression is downregulated during postproliferative maturation.47 Interestingly, VEGFR1 expression was detected on both CD49d+ human and mouse neutrophils, which has not previously been shown, supporting the concept that these neutrophils can be specifically activated by VEGF-A released from hypoxic sites. Moreover, the identified CD49d+VEGFR1+ neutrophils expressed high levels of CXCR4. The specific expression of VEGFR1 and CD49d on this neutrophil subpopulation is crucial for their recruitment thus enabling specific targeting of these cells to modify angiogenesis.
The existence of a CD49d/VEGFR1/CXCR4 triple-positive neutrophil population lends support to the following model for neutrophil extravasation in response to VEGF-A: (1) CD49d allows sustained adhesion to endothelial cells, (2) VEGF-A activates neutrophil VEGFR1-stimulating chemokinesis, and (3) CXCL12 released from tissue activates neutrophil CXCR4-promoting recruitment. These effects in combination with VEGFR2-dependent effects on endothelial cell adhesion molecules and chemokine upregulation will eventually result in neutrophil transmigration.
In conclusion, this study identified, in the circulation of mice and humans, a hitherto uncharacterized preexisting and distinct CD49d+VEGFR1highCXCR4high neutrophil population with proangiogenic properties. Recruitment of these neutrophils to tissue by VEGF-A involves both VEGFR1 and VEGFR2 in a coordinated manner and is dependent on the VLA-4 integrin. When recruitment of the identified proangiogenic neutrophils was inhibited, angiogenesis was significantly impaired in vivo. Importantly, these new insights might be useful drug targets for the specific modulation of angiogenesis without compromising the immune response to pathogens.
Authorship
Contribution: S.M., G.C., and M.W. designed and performed the experiments, acquired, analyzed and interpreted data, and wrote the manuscript; E.V., C.S., K.G., and F.B. designed and performed the experiments, and acquired and analyzed data; C.H.H. performed the experiments, and acquired and analyzed data; A.G., J.L., and S.W. performed the experiments; M.S. contributed the mice; L.C.-W. designed experiments and interpreted data; P.G. designed the experiments; J.K. designed the experiments, interpreted data, and wrote the manuscript; and M.P. initiated, designed, and supervised the study, designed the experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mia Phillipson, Department of Medical Cell Biology, Uppsala University, P.O. Box 571, 75123 Uppsala, Sweden; e-mail: mia.phillipson@mcb.uu.se.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Malin Thyselius, Department of Medical Cell Biology, Uppsala University, for her devotion in analyzing neutrophil recruitment to grafts.
This work was supported by grants from the Swedish Research Council, the Royal Swedish Academy of Sciences, Magnus Bergvalls Foundation, the Swedish Society for Medical Research, the Swedish Diabetes Foundation, Lars Hiertas Foundation, Harald and Greta Jeanssons Foundation, Åke Wibergs Foundation, the Ragnar Söderberg foundation, and the Knut and Alice Wallenberg Foundation (M.P. and L.C.-W.), and a Grant-in-Aid Special Project Research on Cancer-Bioscience grant (17014020) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
Author notes
S.M., G.C., and E.V. contributed equally to this work.

![Figure 1. VEGF-A rapidly induces recruitment of circulating neutrophils to tissue in a VEGFR1- and VEGFR2-dependent manner. VEGF-A-induced leukocyte (A) intravascular adhesion and (B) emigration occur within 30 minutes after onset of VEGF-A superperfusion of the cremaster muscle (control [0 nM], n = 4; VEGF-A 0.2 nM, n = 4; VEGF-A 0.4 nM, n = 4; VEGF-A 2 nM, n = 6; and VEGF-A 20 nM, n = 6). #P < .05 compared with basal values within the same group; *P < .05 compared with control group at the same time point. Leukocyte subsets were differentiated by using CX3CR1GFP/GFP mice and fluorescently tagged anti-Ly6G mAb (clone 1A8). (C) One frame from time 30 minutes in a video recording is shown. Scale bar = 30 μm. Postcapillary venule outlined with dashed lines. (D) The leukocytes recruited after 90 minutes of VEGF-A (2 nM) superperfusion were neutrophils (Ly6G+) and not monocytes (CX3CR1+; control, n = 3; VEGF-A, n = 6). The VEGF-A-induced neutrophil recruitment to muscle was completely inhibited by neutralizing antibodies directed toward either VEGFR1 (clone MF1) or VEGFR2 (clone DC101). (E) Adherent cells and (F) emigrated cells (n = 5 per treatment group). *P < .05 compared with control group at the same time point. (G) Mouse peripheral blood neutrophils expressed levels of VEGFR1 transcripts similar to those in endothelial cells. Relative expression of mRNA presented in arbitrary units (A.U.). Semiquantitative reverse-transcription polymerase chain reaction (n = 11 mice) from 3 independent trials. (H) VEGFR1 and VEGFR2 protein expressions were confirmed on circulating human neutrophils (n = 8) by flow cytometry (isotype in black). (I) Direct stimulation of human neutrophils by either VEGF-A (2 nM) or VEGF-B (5 nM) induced signal transduction via VEGFR1 (phosphorylation of ERK [pERK] compared to total Erk [tErk]; western blot from 4 independent trials).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/17/10.1182_blood-2015-03-631572/4/m_2016f1.jpeg?Expires=1765941252&Signature=pNQ5XEQSX7XVLZ-ZaV5pnAxyqfr7p54bcDDbFu93FAff2mE58IY5MFu~lm1iRaGZfDxAcqRalGQ5KE5DKnySatjXHZCugpZVsKLf0NwIKj1dCfI8sD5H9Bz2U7cafzCk~ZwaZ87pI79fuSe96jsSWuJhT4EvBzmqwCGXBUzAZfsYvER6MUVSbHnSKXO5m~bMRvm4GJSh6z602G93EKRF02g2dJuug151yG3V0YM8Nt4WczGdFIRz0Pf5ev~XcVGCDgY8kMRuo~Fz2cLITO6W2OfaI-jRKkLRNQWLhoAONYNeqyUQzEKL9JL2NU1JIykcQpqtz7QuWyhvOVI4nqX3HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal