Key Points
Patients with early-stage extranodal nasal-type NKTCL were classified as low risk or high risk using 5 independent prognostic factors.
Risk-adapted therapy of RT alone for the low-risk group and RT consolidated by CT for the high-risk group proved the most effective treatment.
Abstract
The optimal combination and sequence of radiotherapy (RT) and chemotherapy (CT) for extranodal nasal-type natural killer/T-cell lymphoma (NKTCL) are not well-defined. The aim of this study was to create a risk-adapted therapeutic strategy for early-stage NKTCL. A total of 1273 early-stage patients from 10 institutions were reviewed. Patients received CT alone (n = 170), RT alone (n = 253), RT followed by CT (n = 209), or CT followed by RT (n = 641). A comprehensive comparative study was performed using multivariable and propensity score-matched analyses. Early-stage NKTCL was classified as low risk or high risk based on 5 independent prognostic factors (stage, age, performance status, lactate dehydrogenase, primary tumor invasion). RT alone and RT with or without CT were more effective than CT alone (5-year overall survival [OS], 69.6% and 67.7% vs 33.9%, P < .001). For low-risk patients, RT alone achieved a favorable OS (88.8%); incorporation of induction or consolidation CT did not provide additional benefit (86.9% and 86.3%). For high-risk patients, RT followed by CT resulted in superior OS (72.2%) compared with induction CT and RT (58.3%, P = .004) or RT alone (59.6%, P = .017). After adjustment, similar significant differences in OS were still observed between treatment groups. New CT regimens provided limited benefit in early-stage NKTCL. Risk-adapted therapy involving RT alone for low-risk patients and RT consolidated by CT for high-risk patients is a viable, effective strategy for early-stage NKTCL.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1517.
Disclosures
Associate Editor Laurie Sehn served as an advisor or consultant for Amgen, Genentech, Gilead, Janssen, Lundbeck, Roche, and Seattle Genetics. The authors and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Discuss independent prognostic factors used to classify patients with early-stage extranodal nasal-type natural killer/T-cell lymphoma (NKTCL) as low risk or high risk.
Describe the efficacy of and recommended strategy for risk-adapted therapy for patients with high-risk, early-stage extranodal nasal-type NKTCL.
Describe the efficacy of risk-adapted therapy for patients with low-risk, early-stage extranodal nasal-type NKTCL.
Release date: September 17, 2015; Expiration date: September 17, 2016
Introduction
In the past 2 decades, extranodal nasal-type natural killer/T-cell lymphoma (NKTCL) has been recognized as a distinct clinicopathologic entity with an aggressive clinical course.1-3 NKTCL can arise within any extranodal organ or tissue, but usually involves the upper aerodigestive tract (UADT) such as the nasal cavity and Waldeyer ring.3-8
Early-stage NKTCL represents 70% to 90% of cases; however, the clinical management of early-stage NKTCL is inconsistent.1-5,7-13 Current guidelines from the National Comprehensive Cancer Network are equivocal regarding the optimal therapy for early-stage NKTCL, and include radiotherapy (RT) alone, sequential chemotherapy (CT) and RT, or concurrent chemoradiotherapy.14 The reported 5-year overall survival (OS) rates for localized NKTCL vary from 30% to 90%,1-13,15-25 reflecting different treatment strategy, disease heterogeneity, and the lack of prognostic factors to enable further tailoring of therapy such as the optimal combination and sequence of RT and CT.
We previously demonstrated that RT is a critical component of curative therapy for early-stage NKTCL4,5,15-17 and leads to excellent locoregional control rates of >90% and 5-year OS of 70% to 90%.4,5,9,10,15-17,25 Other studies reported similar improvement after upfront RT over CT alone.1,18-20 However, this advantage needs to be validated in a multi-institution setting. On the other hand, although previous studies showed that adding CT to RT provided no survival benefit for early-stage disease,1,4,10,12,18,21-25 most patients in these studies received combined modality therapy (CMT) with different sequences and combinations of CT. However, the benefit of adding CT is difficult to assess in a small sample of patients; we hypothesize that adding CT to RT may provide a greater survival improvement for high-risk patients. Other recent studies demonstrated promising results for dose-intensity adjustments and new CT regimens in refractory or advanced NKTCL.26,27 With the development of more effective systemic therapies, the additive effect of CT regimens combined with RT for early-stage NKTCL remains of interest.
Using a large cohort of patients with NKTCL from several institutions, we conducted a comprehensive analysis to stratify patients with early-stage disease into different risk categories, compare the efficacy of RT and CT, and finally optimize a risk-adapted therapeutic strategy.
Patients and methods
Patient eligibility
A total of 1273 patients with previously untreated NKTCL at 10 Chinese institutions were reviewed between 2000 and 2011. Eligibility requirements included the typical histological and immunophenotypic features of NKTCL (World Health Organization classification), stage I and II disease (Ann Arbor staging system), and complete clinicopathologic and follow-up information. Patients underwent standard staging procedures with routine physical and endoscopic examination; biochemistry; computed tomography scans and/or magnetic resonance imaging of the head and neck, chest, abdomen, and pelvis; and a bone marrow examination. Positron emission tomography was recommended but not mandatory. Primary tumor invasion (PTI) was defined as the presence of primary disease that extended into neighboring structures or organs (eg, primary tumor in the nasal cavity with extension of the paranasal sinuses and/or nasopharynx) or the involvement of multiple, contiguous primary sites (eg, primary tumor involving the nasopharynx and oropharynx; primary tumor involving the nasal cavity, nasopharynx, and oropharynx), regardless of the stage or primary site. This project was approved by our institutional review board and conducted in accordance with the Declaration of Helsinki.
Treatment
Because of poor consensus, treatment options varied between and within institutions, mainly depending on the physician choice. Patients received CT alone (n = 170), RT alone (n = 253), RT followed by CT (RT + CT, n = 209), or CT followed by RT (CT + RT, n = 641). RT included extended field or extended involved field encompassing the primary tumor and adjacent regions at a radical dose of 50 Gy, with a 6- to 10-Gy boost to residual disease.15,16 Median dose was 50 Gy (range, 36-74 Gy; dose per fraction, 1.8-2 Gy). Of patients receiving CT, 826 (81.0%) received cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or CHOP-like regimens (old regimens), whereas 194 (19.0%) received l-asparaginase–based (n = 126) or gemcitabine-based (n = 68) regimens (new regimens), such as dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide; gemcitabine, cisplatin, and dexamethasone; or dexamethasone, ifosfamide, methotrexate, and gemcitabine. The median number of CT cycles was 4 (range, 1-14) for all patients: 4 (range, 1-9) for patients with CT alone, 3 (range, 1-14) for patients with CT + RT, and 4 (range, 1-9) for patients with RT + CT.
Statistical analyses
Cox proportional hazards regression model was performed to identify independent risk factors for OS in stage I and II patients. Age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), primary site, B symptoms, lactate dehydrogenase (LDH), stage, PTI, and treatment were included as covariates in multivariate analysis. Propensity score–matched (PSM) analysis was conducted to mirror randomized study design and generate comparable study arms; 1:1 patient matching without replacement was used to pair each patient receiving RT alone with another receiving CT only or CMT whose propensity score was within the designated caliper size. After PSM, baseline covariates and survival rates were compared between treatment groups.
OS and progression-free survival (PFS) were defined as described previously,4-6 assessed with the Kaplan-Meier product limit method, and compared using the log-rank test. When detecting nonproportional hazards, a better estimate of treatment effect was provided by the restricted mean survival time (RMST) for comparison of new and old CT regimens.28 Cox proportional hazards regression was performed using rms package and RMST determined using surv2sampleComp package in R, version 3.0.2 (http://www.r-project.org/). PSM was performed with Stata12; other analyses with IBM SPSS Statistics, version 20.0.
Results
Patient characteristics
Clinical characteristics and survival rates are presented in Table 1. Median age was 43 years (range, 9-87); male:female ratio was 2.26:1. Most patients had good PS and primary disease in the UADT. Elevated LDH was present in 31.7% of patients, 40.0% had B symptoms, PTI was observed in 54.1%, and the majority (74.4%) had stage I disease.
Univariate analysis of the association between clinical characteristics and survival outcomes for all patients with early-stage NKTCL
| . | . | 5-y OS . | . | 5-y PFS . | . |
|---|---|---|---|---|---|
| Characteristic . | No. (%) . | % (95% CI) . | P . | % (95% CI) . | P . |
| Sex | .373 | .811 | |||
| Male | 882 (69.3) | 62.4 (58.5-66.1) | 54.1 (50.4-57.8) | ||
| Female | 391 (30.7) | 66.6 (60.9-71.6) | 55.8 (50.1-61.1) | ||
| Age (y) | <.001 | .022 | |||
| ≤60 | 1099 (86.3) | 65.4 (62.0-68.6) | 55.5 (52.2-58.8) | ||
| >60 | 174 (13.7) | 52.7 (43.4-61.2) | 48.7 (39.8-56.9) | ||
| B symptoms | .160 | .019 | |||
| Yes | 509 (40.0) | 60.9 (55.0-65.4) | 50.6 (53.2-61.0) | ||
| No | 764 (60.0) | 65.5 (61.4-69.2) | 57.2 (44.7-57.5) | ||
| Elevated LDH | <.001 | <.001 | |||
| Yes | 403 (31.7) | 55.9 (50.2-61.2) | 48.7 (43.4-53.9) | ||
| No | 870 (68.3) | 67.7 (63.9-71.2) | 57.4 (53.5-61.1) | ||
| ECOG PS | <.001 | <.001 | |||
| 0-1 | 1202 (94.4) | 65.4 (62.2-68.5) | 56.3 (53.1-59.4) | ||
| ≥2 | 71 (5.6) | 36.6 (24.3-48.9) | 28.0 (17.3-39.6) | ||
| Primary location | .907 | <.001 | |||
| UADT | 1260 (99.0) | 63.7 (60.5-66.7) | 55.1 (52.0-58.2) | ||
| Extra-UADT | 13 (1.0) | 68.2 (29.7-88.6) | 0 | ||
| Ann Arbor stage | <.001 | <.001 | |||
| I | 947 (74.4) | 67.6 (64.0-71.0) | 58.3 (54.6-61.7) | ||
| II | 326 (25.6) | 51.3 (44.7-57.5) | 44.0 (37.8-50.1) | ||
| PTI | <.001 | <.001 | |||
| Yes | 689 (54.1) | 53.0 (48.4-57.4) | 45.2 (40.9-49.4) | ||
| No | 584 (45.9) | 75.9 (71.7-79.6) | 65.6 (61.1-69.7) | ||
| Risk group | <.001 | <.001 | |||
| Low | 298 (23.4) | 86.6 (81.5-90.3) | 73.3 (66.9-78.6) | ||
| High | 975 (76.6) | 56.9 (53.3-60.6) | 49.3 (46.6-53.8) |
| . | . | 5-y OS . | . | 5-y PFS . | . |
|---|---|---|---|---|---|
| Characteristic . | No. (%) . | % (95% CI) . | P . | % (95% CI) . | P . |
| Sex | .373 | .811 | |||
| Male | 882 (69.3) | 62.4 (58.5-66.1) | 54.1 (50.4-57.8) | ||
| Female | 391 (30.7) | 66.6 (60.9-71.6) | 55.8 (50.1-61.1) | ||
| Age (y) | <.001 | .022 | |||
| ≤60 | 1099 (86.3) | 65.4 (62.0-68.6) | 55.5 (52.2-58.8) | ||
| >60 | 174 (13.7) | 52.7 (43.4-61.2) | 48.7 (39.8-56.9) | ||
| B symptoms | .160 | .019 | |||
| Yes | 509 (40.0) | 60.9 (55.0-65.4) | 50.6 (53.2-61.0) | ||
| No | 764 (60.0) | 65.5 (61.4-69.2) | 57.2 (44.7-57.5) | ||
| Elevated LDH | <.001 | <.001 | |||
| Yes | 403 (31.7) | 55.9 (50.2-61.2) | 48.7 (43.4-53.9) | ||
| No | 870 (68.3) | 67.7 (63.9-71.2) | 57.4 (53.5-61.1) | ||
| ECOG PS | <.001 | <.001 | |||
| 0-1 | 1202 (94.4) | 65.4 (62.2-68.5) | 56.3 (53.1-59.4) | ||
| ≥2 | 71 (5.6) | 36.6 (24.3-48.9) | 28.0 (17.3-39.6) | ||
| Primary location | .907 | <.001 | |||
| UADT | 1260 (99.0) | 63.7 (60.5-66.7) | 55.1 (52.0-58.2) | ||
| Extra-UADT | 13 (1.0) | 68.2 (29.7-88.6) | 0 | ||
| Ann Arbor stage | <.001 | <.001 | |||
| I | 947 (74.4) | 67.6 (64.0-71.0) | 58.3 (54.6-61.7) | ||
| II | 326 (25.6) | 51.3 (44.7-57.5) | 44.0 (37.8-50.1) | ||
| PTI | <.001 | <.001 | |||
| Yes | 689 (54.1) | 53.0 (48.4-57.4) | 45.2 (40.9-49.4) | ||
| No | 584 (45.9) | 75.9 (71.7-79.6) | 65.6 (61.1-69.7) | ||
| Risk group | <.001 | <.001 | |||
| Low | 298 (23.4) | 86.6 (81.5-90.3) | 73.3 (66.9-78.6) | ||
| High | 975 (76.6) | 56.9 (53.3-60.6) | 49.3 (46.6-53.8) |
CI, confidence interval.
Risk stratification and survival
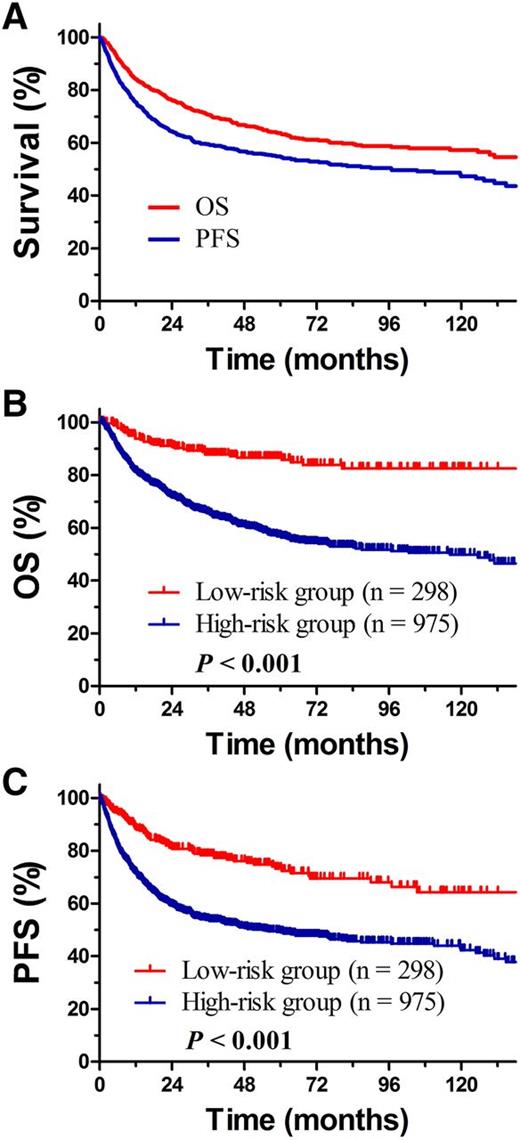
The prognostic significance of clinical features for OS and PFS was evaluated for all early-stage patients (Table 1). In accordance with our previous study,29 age, ECOG PS, stage, LDH, and PTI significantly influenced OS in multivariate analysis (Table 2). Treatment strategy was also an independent prognostic factor for OS. CT alone provided the poorest outcome (hazard ratio 3.707; P < .001). Within the median follow-up of 53 months for surviving patients, 5-year OS and PFS for all patients were 63.7% and 54.9% (Figure 1A).
Multivariable analysis of the association between clinical variables and treatment with OS for all patients with early-stage NKTCL
| . | OS . | ||
|---|---|---|---|
| Variable . | HR . | 95% CI . | P . |
| Ann Arbor stage (II vs I) | 1.551 | 1.258-1.912 | <.001 |
| PTI (yes vs no) | 1.951 | 1.578-2.411 | <.001 |
| Age (>60 vs ≤60 y) | 1.645 | 1.273-2.126 | .002 |
| Elevated LDH level (yes vs no) | 1.240 | 1.013-1.518 | .037 |
| ECOG PS (≥2 vs 0-1) | 1.935 | 1.401-2.671 | .009 |
| Treatment modality | |||
| RT + CT | |||
| CT + RT | 1.481 | 1.081-2.027 | .014 |
| RT alone | 1.561 | 1.072-2.273 | .020 |
| CT alone | 3.707 | 2.599-5.288 | <.001 |
| . | OS . | ||
|---|---|---|---|
| Variable . | HR . | 95% CI . | P . |
| Ann Arbor stage (II vs I) | 1.551 | 1.258-1.912 | <.001 |
| PTI (yes vs no) | 1.951 | 1.578-2.411 | <.001 |
| Age (>60 vs ≤60 y) | 1.645 | 1.273-2.126 | .002 |
| Elevated LDH level (yes vs no) | 1.240 | 1.013-1.518 | .037 |
| ECOG PS (≥2 vs 0-1) | 1.935 | 1.401-2.671 | .009 |
| Treatment modality | |||
| RT + CT | |||
| CT + RT | 1.481 | 1.081-2.027 | .014 |
| RT alone | 1.561 | 1.072-2.273 | .020 |
| CT alone | 3.707 | 2.599-5.288 | <.001 |
HR, hazard ratio.
OS and PFS for all patients. (A) Early-stage NKTCL, (B) OS, and (C) PFS for patients with early-stage NKTCL stratified into the low- and high-risk groups.
OS and PFS for all patients. (A) Early-stage NKTCL, (B) OS, and (C) PFS for patients with early-stage NKTCL stratified into the low- and high-risk groups.
To establish risk-adapted therapy, early-stage patients were stratified as low- and high-risk groups based on 5 independent risk factors (age >60 years, ECOG ≥2, stage II disease, elevated LDH, PTI) unrelated to treatment (Table 2). Low-risk early-stage patients (defined as no risk factors, 23.4%) had significantly better outcome than high-risk early-stage patients (defined as ≥1 risk factor, 76.6%), with 5-year OS and PFS rates of 86.6% and 73.3% for the low-risk group and 56.9% (P < .001, Figure 1B) and 49.3% (P < .001, Figure 1C) for the high-risk group. The 5-year relapse rate was 22.1% for low-risk group and 35.3% for high-risk group (P < .001).
Primary RT improves survival
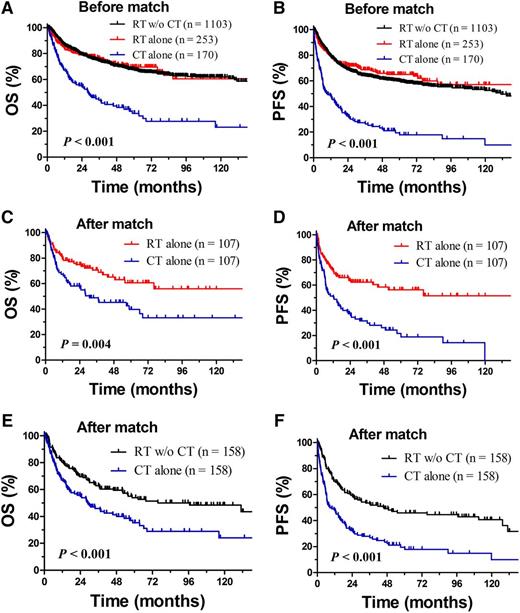
First, we evaluated the efficacy of RT vs CT only. In the unadjusted population, patients treated with CT alone tended to have more risk factors than those treated with RT (Table 3). RT achieved a much better outcome than CT alone; CT alone had a very poor outcome. The 5-year OS and PFS rates were only 33.9% and 19.4% for CT alone, compared with 69.6% (P < .001) and 65.1% (P < .001) for RT alone and 67.7% (P < .001) and 59.8% (P < .001) for RT with or without CT (Figure 2A-B). The 5-year relapse rate was 68.6% for CT alone and 28.2% for RT with or without CT (P < .001). Similar differences between RT and CT alone were also observed when patients were stratified as the low- and high-risk groups (data not shown).
Clinical characteristics of patients with early-stage NKTCL before and after PSM stratification by treatment
| . | RT alone . | CT alone . | . | RT with or without CT . | CT alone . | . |
|---|---|---|---|---|---|---|
| . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . |
| Before match | ||||||
| Total number | 253 | 170 | 1103 | 170 | ||
| Gender, male | 169 (66.8) | 121 (71.2) | .342 | 761 (69.0) | 121 (71.2) | .566 |
| Age, >60 y | 51 (20.2) | 28 (16.5) | .340 | 146 (13.2) | 28 (16.5) | .253 |
| B symptoms | 62 (24.5) | 80 (47.1) | <.001 | 429 (38.9) | 80 (47.1) | .043 |
| Elevated LDH | 58 (22.9) | 66 (38.8) | <.001 | 337 (30.6) | 66 (38.8) | .031 |
| ECOG ≥2 | 8 (3.2) | 21 (12.4) | <.001 | 50 (4.5) | 21 (12.4) | <.001 |
| UADT | 252 (99.6) | 163 (95.9) | .008 | 1097 (99.5) | 163 (95.9) | .001 |
| Stage II | 42 (16.6) | 61 (35.9) | <.001 | 265 (24.0) | 61 (35.9) | .001 |
| PTI | 100 (39.5) | 104 (61.2) | <.001 | 585 (53.0) | 104 (61.2) | .047 |
| After match | ||||||
| Total number | 107 | 107 | 158 | 158 | ||
| Gender, male | 77 (72.0) | 75 (70.1) | .763 | 119 (75.3) | 111 (70.3) | .312 |
| Age, >60 y | 16 (15.0) | 17 (15.9) | .850 | 28 (17.7) | 25 (15.8) | .651 |
| B symptoms | 38 (35.5) | 37 (34.6) | .886 | 85 (53.8) | 75 (47.5) | .261 |
| Elevated LDH | 33 (30.8) | 32 (29.9) | .882 | 60 (38.0) | 64 (40.5) | .645 |
| ECOG ≥2 | 3 (2.8) | 1 (0.9) | .621 | 12 (7.6) | 12 (7.6) | 1.000 |
| UADT | 106 (99.1) | 105 (98.1) | 1.000 | 154 (97.5) | 154 (97.5) | 1.000 |
| Stage II | 34 (31.8) | 35 (32.7) | .884 | 53 (33.5) | 56 (35.4) | .723 |
| PTI | 64 (59.8) | 64 (59.8) | 1.000 | 98 (63.0) | 101 (63.9) | .727 |
| . | RT alone . | CT alone . | . | RT with or without CT . | CT alone . | . |
|---|---|---|---|---|---|---|
| . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . |
| Before match | ||||||
| Total number | 253 | 170 | 1103 | 170 | ||
| Gender, male | 169 (66.8) | 121 (71.2) | .342 | 761 (69.0) | 121 (71.2) | .566 |
| Age, >60 y | 51 (20.2) | 28 (16.5) | .340 | 146 (13.2) | 28 (16.5) | .253 |
| B symptoms | 62 (24.5) | 80 (47.1) | <.001 | 429 (38.9) | 80 (47.1) | .043 |
| Elevated LDH | 58 (22.9) | 66 (38.8) | <.001 | 337 (30.6) | 66 (38.8) | .031 |
| ECOG ≥2 | 8 (3.2) | 21 (12.4) | <.001 | 50 (4.5) | 21 (12.4) | <.001 |
| UADT | 252 (99.6) | 163 (95.9) | .008 | 1097 (99.5) | 163 (95.9) | .001 |
| Stage II | 42 (16.6) | 61 (35.9) | <.001 | 265 (24.0) | 61 (35.9) | .001 |
| PTI | 100 (39.5) | 104 (61.2) | <.001 | 585 (53.0) | 104 (61.2) | .047 |
| After match | ||||||
| Total number | 107 | 107 | 158 | 158 | ||
| Gender, male | 77 (72.0) | 75 (70.1) | .763 | 119 (75.3) | 111 (70.3) | .312 |
| Age, >60 y | 16 (15.0) | 17 (15.9) | .850 | 28 (17.7) | 25 (15.8) | .651 |
| B symptoms | 38 (35.5) | 37 (34.6) | .886 | 85 (53.8) | 75 (47.5) | .261 |
| Elevated LDH | 33 (30.8) | 32 (29.9) | .882 | 60 (38.0) | 64 (40.5) | .645 |
| ECOG ≥2 | 3 (2.8) | 1 (0.9) | .621 | 12 (7.6) | 12 (7.6) | 1.000 |
| UADT | 106 (99.1) | 105 (98.1) | 1.000 | 154 (97.5) | 154 (97.5) | 1.000 |
| Stage II | 34 (31.8) | 35 (32.7) | .884 | 53 (33.5) | 56 (35.4) | .723 |
| PTI | 64 (59.8) | 64 (59.8) | 1.000 | 98 (63.0) | 101 (63.9) | .727 |
Comparison of OS and PFS between RT and CT alone. OS (A) and PFS (B) for patients with early-stage NKTCL after CT alone, RT alone, and RT with or without CT (RT w/o RT) before match stratification; OS (C) and PFS (D) after RT alone and CT alone after match stratification; and OS (E) and PFS (F) after RT with or without CT and CT alone after match stratification.
Comparison of OS and PFS between RT and CT alone. OS (A) and PFS (B) for patients with early-stage NKTCL after CT alone, RT alone, and RT with or without CT (RT w/o RT) before match stratification; OS (C) and PFS (D) after RT alone and CT alone after match stratification; and OS (E) and PFS (F) after RT with or without CT and CT alone after match stratification.
After adjustment by PSM, prognostic factors were comparable between treatment groups (Table 3), and RT still resulted in significantly better survival than CT alone. The 5-year OS and PFS rates were 60.6% and 56.3% for RT alone and 39.9% (P = .004, Figure 2C) and 21.7% (P < .001, Figure 2D) for CT alone. The corresponding OS and PFS rates for RT with or without CT were 53.9% and 45.9%, respectively, and 35.2% (P < .001, Figure 2E) and 19.5% (P < .001, Figure 2F) for CT alone.
Excellent outcome for RT but no additional benefit from CT in low-risk patients
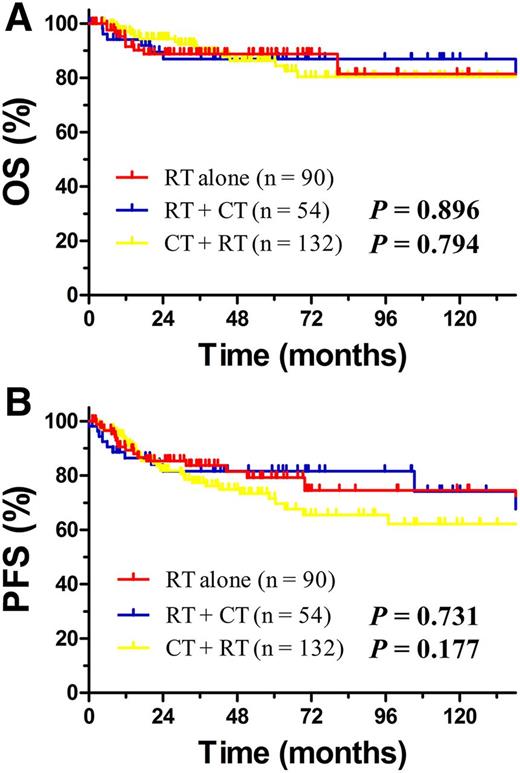
We subsequently evaluated whether adding CT to RT modified the outcome in different risk groups. For low-risk patients, RT achieved very favorable long-term survival. Neither induction nor consolidation CT provided additional survival benefit; the 5-year OS rate was 88.8% for RT alone compared with 86.9% (P = .896) for RT + CT and 86.3% (P = .794) for CT + RT, respectively (Figure 3A). The corresponding PFS rate was 79.2% for RT alone, 81.6% for RT + CT (P = .731), and 71.5% for CT + RT (P = .177, Figure 3B). The 5-year relapse rate was 18.8% for RT alone, 10.3% for RT + CT, and 23.8% for CT + RT (P = .255), respectively. PSM analysis was not performed because no low-risk patient had any adverse factor.
Comparison of OS and PFS between RT alone, RT + CT and CT + RT for low-risk early-stage patients. OS (A) and PFS (B) for low-risk patients with early-stage NKTCL stratified by RT alone, RT + CT, and CT + RT.
Comparison of OS and PFS between RT alone, RT + CT and CT + RT for low-risk early-stage patients. OS (A) and PFS (B) for low-risk patients with early-stage NKTCL stratified by RT alone, RT + CT, and CT + RT.
RT followed by CT improves survival in high-risk patients
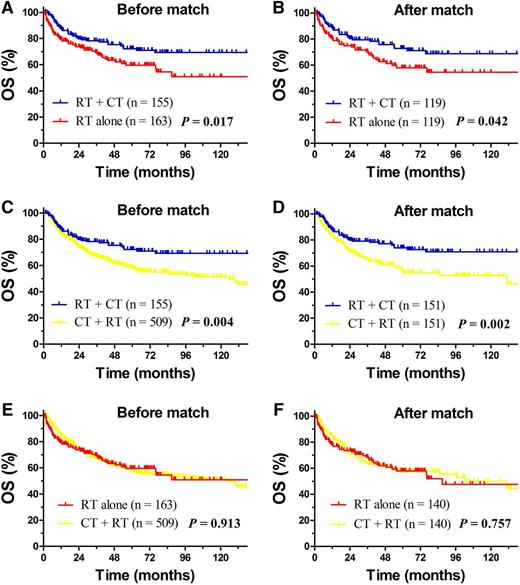
To further define the additional benefit of CT and optimize the RT/CT sequence for high-risk patients, we compared the outcomes between RT alone, CT + RT, and RT + CT. In the unadjusted population, older patients tended to receive RT alone, whereas patients with stage II disease and B symptoms were more likely to receive CMT (Table 4). RT followed by CT significantly improved survival compared with RT alone or induction CT and RT; the 5-year OS rate was 72.2% for RT + CT compared with 59.6% for RT alone (P = .017, Figure 4A) and 58.3% for CT + RT (P = .004, Figure 4C), with comparable OS for the latter 2 groups (P = .913, Figure 4E). The corresponding relapse rate was 23.1% for RT + CT, 30.3% for RT alone, and 34.0% for CT + RT, respectively.
Clinical characteristics of high-risk patients with early-stage NKTCL before and after PSM stratification by treatment
| . | RT + CT . | RT alone . | . | RT + CT . | CT + RT . | . | RT alone . | CT + RT . | . |
|---|---|---|---|---|---|---|---|---|---|
| . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . |
| Before match | |||||||||
| Total | 155 | 163 | 155 | 509 | 163 | 509 | |||
| Gender, male | 111 (71.6) | 113 (69.3) | .655 | 111 (71.6) | 363 (71.3) | .943 | 113 (69.3) | 363 (71.3) | .626 |
| Age, >60 y | 20 (12.9) | 51 (31.3) | <.001 | 20 (12.9) | 75 (14.7) | .569 | 51 (31.3) | 75 (14.7) | <.001 |
| B symptoms | 69 (44.5) | 43 (26.4) | .001 | 69 (44.5) | 240 (47.2) | .565 | 43 (26.4) | 240 (47.2) | <.001 |
| Elevated LDH | 59 (38.1) | 58 (35.6) | .646 | 59 (38.1) | 220 (43.2) | .255 | 58 (35.6) | 220 (43.2) | .085 |
| ECOG ≥2 | 12 (7.7) | 8 (4.9) | .298 | 12 (7.7) | 30 (5.9) | .408 | 8 (4.9) | 30 (5.9) | .635 |
| UADT | 155 (100) | 163 (100) | 1.000 | 155 (100) | 506 (99.4) | 1.000 | 163 (100) | 506 (99.4) | 1.000 |
| Stage II | 40 (25.8) | 42 (25.8) | .994 | 40 (25.8) | 183 (36.0) | .019 | 42 (25.8) | 183 (36.0) | .016 |
| PTI | 114 (73.5) | 100 (61.3) | .020 | 114 (73.5) | 371 (72.9) | .871 | 100 (61.3) | 371 (72.9) | .005 |
| After match | |||||||||
| Total | 119 | 119 | 151 | 151 | 140 | 140 | |||
| Gender, male | 86 (72.3) | 80 (67.2) | .397 | 109 (72.2) | 114 (75.5) | .513 | 98 (70.0) | 98 (70.0) | 1.000 |
| Age, >60 y | 18 (15.1) | 18 (15.1) | 1.000 | 18 (11.9) | 18 (11.9) | 1.000 | 30 (21.4) | 27 (19.3) | .656 |
| B symptoms | 48 (40.3) | 31 (26.1) | .019 | 66 (43.7) | 66 (43.7) | 1.000 | 40 (28.6) | 37 (26.4) | .688 |
| Elevated LDH | 43 (36.1) | 43 (36.1) | 1.000 | 55 (36.4) | 57 (36.4) | 1.000 | 52 (37.1) | 52 (37.1) | 1.000 |
| ECOG ≥2 | 5 (4.2) | 5 (4.2) | 1.000 | 8 (5.3) | 8 (5.3) | 1.000 | 4 (2.9) | 4 (2.9) | 1.000 |
| UADT | 119 (100) | 119 (100) | 1.000 | 151 (100) | 151 (100) | 1.000 | 140 (100) | 140 (100) | 1.000 |
| Stage II | 28 (23.5) | 28 (23.5) | 1.000 | 39 (25.5) | 39 (25.5) | 1.000 | 40 (28.6) | 40 (28.6) | 1.000 |
| PTI | 84 (70.6) | 84 (70.6) | 1.000 | 111 (73.5) | 111 (73.5) | 1.000 | 99 (70.7) | 99 (70.7) | 1.000 |
| . | RT + CT . | RT alone . | . | RT + CT . | CT + RT . | . | RT alone . | CT + RT . | . |
|---|---|---|---|---|---|---|---|---|---|
| . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . |
| Before match | |||||||||
| Total | 155 | 163 | 155 | 509 | 163 | 509 | |||
| Gender, male | 111 (71.6) | 113 (69.3) | .655 | 111 (71.6) | 363 (71.3) | .943 | 113 (69.3) | 363 (71.3) | .626 |
| Age, >60 y | 20 (12.9) | 51 (31.3) | <.001 | 20 (12.9) | 75 (14.7) | .569 | 51 (31.3) | 75 (14.7) | <.001 |
| B symptoms | 69 (44.5) | 43 (26.4) | .001 | 69 (44.5) | 240 (47.2) | .565 | 43 (26.4) | 240 (47.2) | <.001 |
| Elevated LDH | 59 (38.1) | 58 (35.6) | .646 | 59 (38.1) | 220 (43.2) | .255 | 58 (35.6) | 220 (43.2) | .085 |
| ECOG ≥2 | 12 (7.7) | 8 (4.9) | .298 | 12 (7.7) | 30 (5.9) | .408 | 8 (4.9) | 30 (5.9) | .635 |
| UADT | 155 (100) | 163 (100) | 1.000 | 155 (100) | 506 (99.4) | 1.000 | 163 (100) | 506 (99.4) | 1.000 |
| Stage II | 40 (25.8) | 42 (25.8) | .994 | 40 (25.8) | 183 (36.0) | .019 | 42 (25.8) | 183 (36.0) | .016 |
| PTI | 114 (73.5) | 100 (61.3) | .020 | 114 (73.5) | 371 (72.9) | .871 | 100 (61.3) | 371 (72.9) | .005 |
| After match | |||||||||
| Total | 119 | 119 | 151 | 151 | 140 | 140 | |||
| Gender, male | 86 (72.3) | 80 (67.2) | .397 | 109 (72.2) | 114 (75.5) | .513 | 98 (70.0) | 98 (70.0) | 1.000 |
| Age, >60 y | 18 (15.1) | 18 (15.1) | 1.000 | 18 (11.9) | 18 (11.9) | 1.000 | 30 (21.4) | 27 (19.3) | .656 |
| B symptoms | 48 (40.3) | 31 (26.1) | .019 | 66 (43.7) | 66 (43.7) | 1.000 | 40 (28.6) | 37 (26.4) | .688 |
| Elevated LDH | 43 (36.1) | 43 (36.1) | 1.000 | 55 (36.4) | 57 (36.4) | 1.000 | 52 (37.1) | 52 (37.1) | 1.000 |
| ECOG ≥2 | 5 (4.2) | 5 (4.2) | 1.000 | 8 (5.3) | 8 (5.3) | 1.000 | 4 (2.9) | 4 (2.9) | 1.000 |
| UADT | 119 (100) | 119 (100) | 1.000 | 151 (100) | 151 (100) | 1.000 | 140 (100) | 140 (100) | 1.000 |
| Stage II | 28 (23.5) | 28 (23.5) | 1.000 | 39 (25.5) | 39 (25.5) | 1.000 | 40 (28.6) | 40 (28.6) | 1.000 |
| PTI | 84 (70.6) | 84 (70.6) | 1.000 | 111 (73.5) | 111 (73.5) | 1.000 | 99 (70.7) | 99 (70.7) | 1.000 |
Comparison of OS between RT + CT, RT alone and CT + RT for high-risk early-stage patients. OS for high-risk patients with early-stage NKTCL after RT + CT or RT alone before (A) and after match (B) stratification; OS after RT + CT and CT + RT before (C) and after (D) match stratification; and OS after RT alone and CT + RT before (E) and after (F) match stratification.
Comparison of OS between RT + CT, RT alone and CT + RT for high-risk early-stage patients. OS for high-risk patients with early-stage NKTCL after RT + CT or RT alone before (A) and after match (B) stratification; OS after RT + CT and CT + RT before (C) and after (D) match stratification; and OS after RT alone and CT + RT before (E) and after (F) match stratification.
PSM adequately balanced clinical variables affecting treatment selection (Table 4). After adjustment, the risk of lymphoma-related death remained significantly lower in high-risk patients receiving RT followed by CT; the 5-year OS rate was 72.8% for RT + CT compared with 57.9% for RT alone (P = .042, Figure 4B) and 57.3% for CT + RT (P = .002, Figure 4D). The OS rate was comparable for RT alone and CT + RT (P = .757, Figure 4F), indicating induction CT provided no additional benefit in high-risk patients.
Limited benefit of new CT regimens
We compared the outcomes for new and old regimens in patients receiving CT alone or CMT. Most clinical characteristics were comparable between groups (Table 5). The overall response rate (complete and partial response) was 77.9% for new regimens and 61.3% for old regimens (P < .001). However, these overall response rates were significantly lower than those after initial RT (93.3%, P < .001). The complete response (CR) rate was 31.6% for new regimens, 25.1% for old regimens (P = .121), and 82.2% for RT (P < .001). Furthermore, the CR rate was 37.6% for l-asparaginase–based regimens, with 45.5% after more than 2 cycles of CT and 21.9% after 1 to 2 cycles of CT, respectively (P = .024).
Clinical characteristics of patients with early-stage NKTCL stratified by treatment and CT regimen
| . | CT alone . | CT + RT . | RT + CT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | New regimens . | Old regimens . | . | New regimens . | Old regimens . | . | New regimens . | Old regimens . | . |
| . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . |
| Total | 39 | 131 | 118 | 523 | 37 | 172 | |||
| Gender, male | 32 (82.1) | 89 (67.9) | .088 | 82 (69.5) | 366 (70.0) | .917 | 22 (59.5) | 122 (70.9) | .172 |
| Age, >60 y | 9 (23.1) | 19 (14.5) | .205 | 15 (12.7) | 60 (11.5) | .705 | 3 (8.1) | 17 (9.9) | 1.000 |
| B symptoms | 21 (53.8) | 59 (45.0) | .333 | 53 (44.9) | 231 (44.2) | .883 | 21 (56.8) | 62 (36.0) | .020 |
| Elevated LDH | 16 (41.0) | 50 (38.2) | .748 | 35 (29.7) | 185 (35.4) | .238 | 13 (35.1) | 46 (26.7) | .304 |
| ECOG ≥2 | 5 (12.8) | 16 (12.2) | 1.000 | 4 (3.4) | 26 (5.0) | .463 | 1 (2.7) | 11 (6.4) | .697 |
| UADT | 1 (2.6) | 6 (4.6) | 1.000 | 1 (0.8) | 4 (0.8) | 1.000 | 37 (100) | 172 (100) | 1.000 |
| Stage II | 15 (38.5) | 46 (35.7) | .702 | 35 (29.7) | 148 (28.3) | .767 | 13 (35.1) | 27 (15.7) | .006 |
| PTI | 17 (43.6) | 87 (66.4) | .010 | 61 (51.7) | 310 (59.3) | .132 | 27 (73.0) | 87 (50.6) | .013 |
| . | CT alone . | CT + RT . | RT + CT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | New regimens . | Old regimens . | . | New regimens . | Old regimens . | . | New regimens . | Old regimens . | . |
| . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . | No. (%) . | No. (%) . | P . |
| Total | 39 | 131 | 118 | 523 | 37 | 172 | |||
| Gender, male | 32 (82.1) | 89 (67.9) | .088 | 82 (69.5) | 366 (70.0) | .917 | 22 (59.5) | 122 (70.9) | .172 |
| Age, >60 y | 9 (23.1) | 19 (14.5) | .205 | 15 (12.7) | 60 (11.5) | .705 | 3 (8.1) | 17 (9.9) | 1.000 |
| B symptoms | 21 (53.8) | 59 (45.0) | .333 | 53 (44.9) | 231 (44.2) | .883 | 21 (56.8) | 62 (36.0) | .020 |
| Elevated LDH | 16 (41.0) | 50 (38.2) | .748 | 35 (29.7) | 185 (35.4) | .238 | 13 (35.1) | 46 (26.7) | .304 |
| ECOG ≥2 | 5 (12.8) | 16 (12.2) | 1.000 | 4 (3.4) | 26 (5.0) | .463 | 1 (2.7) | 11 (6.4) | .697 |
| UADT | 1 (2.6) | 6 (4.6) | 1.000 | 1 (0.8) | 4 (0.8) | 1.000 | 37 (100) | 172 (100) | 1.000 |
| Stage II | 15 (38.5) | 46 (35.7) | .702 | 35 (29.7) | 148 (28.3) | .767 | 13 (35.1) | 27 (15.7) | .006 |
| PTI | 17 (43.6) | 87 (66.4) | .010 | 61 (51.7) | 310 (59.3) | .132 | 27 (73.0) | 87 (50.6) | .013 |
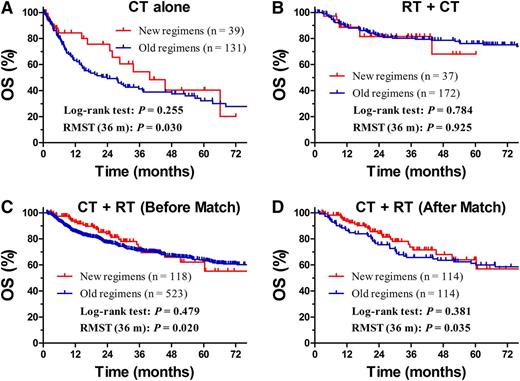
For patients treated with CT alone, the OS curves for the new and old regimens were not significantly different (P = .255, log-rank test; Figure 5A). RMST (OS) up to 36 months was 27.5 months for new regimens and 21.7 months for old regimens (difference, 5.8 months; ratio, 1.27; P = .030). For RT followed by CT, the OS rates for the new and old regimens were not significantly different in either the log-rank test or RMST analysis (Figure 5B). For CT followed by RT, the OS rates for the 2 regimens were not significantly different (P = .479, log-rank test): estimated RMST up to 36 months was 31.9 months for new regimens and 29.9 months for old regimens (difference, 2 months; ratio, 1.07; P = .020, Figure 5C). After adjustment by PSM, similarly different RMST was observed between both regimens (P = .035, Figure 5D).
Comparison of OS between new regimens and old regimens. OS for patients with early-stage NKTCL receiving CT alone (A) or RT + CT (B) stratified by the new or old regimens before match stratification. OS for patients with early-stage NKTCL who received CT + RT stratified by the new or old regimens before (C) and after match (D) stratification.
Comparison of OS between new regimens and old regimens. OS for patients with early-stage NKTCL receiving CT alone (A) or RT + CT (B) stratified by the new or old regimens before match stratification. OS for patients with early-stage NKTCL who received CT + RT stratified by the new or old regimens before (C) and after match (D) stratification.
Discussion
The optimal combination and sequence of RT and CT for early-stage NKTCL has not been defined. This multi-institution study assessed the treatment outcomes of risk-adapted therapy in the largest cohort of patients reported to date. Patients with early-stage NKTCL were classified into low- or high-risk groups using 5 independent prognostic factors. We have demonstrated that RT is an effective treatment of early-stage NKTCL and is significantly better than CT alone. For low-risk patients, RT alone achieved a favorable outcome; induction or consolidation CT did not provide additional benefit. For high-risk patients, RT followed by CT resulted in superior OS compared with RT alone or induction CT and RT. Furthermore, following reports of improved OS and PFS after RT for early-stage NKTCL,4-6,9-13 this is the first study to confirm the additional benefit of consolidation CT in high-risk patients. Additionally, new CT regimens provide only limited benefit in early-stage NKTCL.
Patients with early-stage NKTCL represent a heterogeneous population with 5-year OS rates ranging from 36.6% to 86.6% (Table 1). As indicated in Table 2, the risk of death is highly variable because of the interactions between clinical characteristics and treatment. However, no previous study has examined the value of risk-adapted therapy based on clinical characteristics in early-stage NKTCL. A nomogram model based on 5 independent prognostic factors has been developed and was validated in our previous study.29 Moreover, in this large cohort of patients with early-stage NKTCL, we developed a new dedicated risk category system according to these risk factors, including age >60 years, elevated LDH, ECOG PS ≥2, stage II, and PTI; these were most significant prognostic factors and criteria for treatment decisions and provided discrimination between low- and high-risk patients.
The rarity and heterogeneity of NKTCL and lack of prospective trial data have resulted in a variety of treatment options, CT regimens, and RT volumes and doses at different institutions.1,3,15-21 Based on experience with diffuse large B-cell lymphoma (DLBCL), early-stage aggressive T-cell lymphoma is traditionally treated with doxorubicin-based CT with or without RT. However, the most common subtypes of peripheral T-cell lymphoma, such as NKTCL and peripheral T-cell lymphoma not otherwise specified, are resistant to CT. The outcomes for CT alone in early-stage NKTCL have been poor (CR, 20%-50%; overall response rate, 50%-70%; 5-year OS, 10%-35%, and even poorer PFS).1-3,18,19,30-33 Similarly, we confirmed the unfavorable prognosis of early-stage patients treated with CT alone—regardless of regimens—and obtained a significant survival improvement after RT (in both multivariable and PSM analysis). In the unadjusted population, 5-year OS was only 33.9% for CT alone compared with 69.6% for RT alone and 67.7% for RT with or without CT. Similar significant differences in OS and PFS between RT and CT alone were also observed in the adjusted population. These results are consistent with other studies that compared survival across different treatments in early-stage NKTCL,1,3,7,18-21,30,33 with reported 5-year OS rates ranging from 50% to 90% after RT vs <30% for CT alone. The striking difference in 5-year OS (>20%) between RT and CT alone suggests RT is an essential treatment of early-stage NKTCL and that CT alone should not be routinely administrated. The high cure rate obtained in this large cohort of patients across a substantial number of institutions demonstrates the efficacy and feasibility of primary RT in early-stage NKTCL.
RT is the backbone of curative intent for early-stage NKTCL; however, the additive effect of CT and optimal RT/CT sequence remains unclear. Generally, CMT is frequently used, with CT mainly preceding RT.1-8 However, most previous studies did not confirm the survival benefit of adding CT to RT in early-stage NKTCL.4,10-12,21,34,35 In light of the low efficacy of CT, the benefit of adding CT to RT may be limited in low-risk patients but greater in high-risk patients. Here, we demonstrate that RT achieved a very favorable outcome, with 5-year OS of approximately 90% for low-risk patients comprising one-quarter of cases of early-stage disease. However, induction or consolidation CT failed to provide additional survival benefit, suggesting that RT alone is a viable option for low-risk patients. For high-risk patients, RT and consolidation CT proved most effective. The additive benefit of CT was only observed in patients treated with RT followed by CT—in that order exclusively—suggesting that delaying RT may negatively impact the effectiveness of treatment. Moreover, CT followed by RT and RT alone had similarly poor outcomes, indicating that induction CT may be ineffective. RT followed by CT provided a 15% increase in 5-year OS (after correcting for treatment selection bias with PSM). Moreover, the limited improvements in initial response and survival provided by the new regimens compared with the old regimens in the CT or CMT groups in this study suggests that the new CT regimens may not be as effective as expected in early-stage NKTCL.26,36-38 Consistently, a recent study reported a better outcome with early RT than late RT in patients treated with l-asparaginase–containing CT and RT.36 On the other hand, high levels of acute toxicity induced by dose-intensity CT,26,27,36 which may delay effective RT, is a critical concern when defining CT/RT sequences. To avoid a delay in RT, several prospective trials applied concurrent chemoradiotherapy, with reported 5-year OS rates of 60% to 73%.37-40 In this large cohort of early-stage patients, the 5-year OS rates after RT alone for the low-risk group (88.8%) or after sequential RT and CT for the high-risk group (72.2%) were superior or comparable to other recent small series of concurrent, sequential, or “sandwich” CT and RT, regardless of the CT regimen.36-44 Therefore, RT followed by optional CT offers the advantage of effective RT and has a more tolerable safety profile4-6,15,16 and may reduce the risk of chemoresistance.
Based on our findings, risk-adapted therapy involving RT alone for low-risk patients and RT consolidated with CT for high-risk patients should be considered the optimal strategy for early-stage NKTCL. This approach is inspired by and mirrors the standard of care for early-stage DLBCL (CT followed by optional RT).45-47 In contrast to DLBCL, NKTCL is resistant to CT but highly sensitive to RT. RT alone achieved similar 5-year OS in early-stage NKTCL as CT alone in early-stage DLBCL (50%-90%)4-6,16-22,45-47 ; however, CT alone achieved a similarly low OS in early-stage NKTCL (10%-35%) as RT alone in early-stage DLBCL (30%-50%).1-3,7,8,48-50 Henceforth, it is logical to reverse the order of CT and RT in initial risk-adapted therapy for early-stage NKTCL.
This retrospective study has some limitations. Although the data confirm important findings regarding improved survival after risk-adapted therapy, the treatments were not randomly assigned. High-risk patients were more likely to receive CMT; therefore, the results may be affected by selection biases. We attempted to circumvent this limitation using PSM to account for prognostic factors. After PSM adjustment, the numbers of patients (n > 100 in each group) were sufficient to compare survival differences between treatment groups. The CR rate with l-asparaginase–based CT in this study was similar to some previous studies,41,51-57 but lower than other studies.26,27,42,58-62 The different CR rates in these studies were probably due to small sample size, different clinical stage included, heterogeneous l-asparaginase–containing regimens and cycles, use of RT, and other unknown factors (see supplemental Table 1 on the Blood Web site). Because a minority of our patients (12%) received new CT regimens and had a shorter follow-up time, incorporation of more effective CT regimens into risk-adapted therapy warrants further investigation. The conclusions on initial risk-adapted RT for early-stage NKTCL may be affected by the use of modern effective CT.
Based on current data, we suggest risk-adapted therapy for early-stage NKTCL: RT alone for low-risk patients and RT followed by CT for high-risk patients. Future prospective studies are required to refine treatment by incorporating more effective CT regimens and novel molecular markers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.-X.L. designed the research; Y.-X.L. and Y.Y. collected and analyzed data; Y.Z., Y.Y., and Y.-X.L. wrote the article; and all authors provided study materials or patients and approved the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ye-Xiong Li, Department of Radiation Oncology, Cancer Hospital and Institute, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100021, P. R. China; e-mail: yexiong12@163.com.
References
Author notes
Y.Y. and Y.Z. contributed equally to this work.