To the editor:
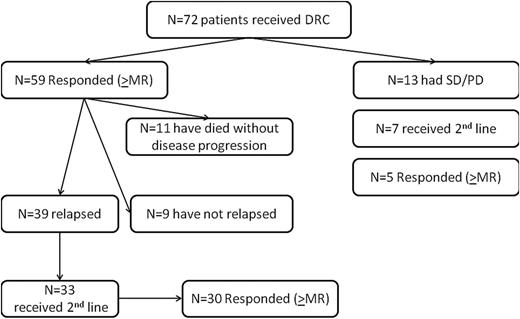
Reports of long-term outcomes of therapies in Waldenström macroglobulinemia (WM) are particularly important given the protracted course of the disease. In a large phase 2 study in 72 patients, using contemporary criteria for diagnosis and initiation of therapy,1,2 primary therapy with dexamethasone, rituximab, and cyclophosphamide (DRC) was active with limited toxicity.3 DRC is one of the most commonly used regimens for symptomatic WM and is evaluated, with or without bortezomib, in the randomized European Consortium for Waldenström Macroglobulinemia (ECWM-1) study (NCT01788020). Herein we present outcomes after a minimum follow-up of 7 years (median 8, range 7 to 10 years), and this is one of the few studies in WM with long-term data. Patients were enrolled between November 2002 and April 2006. Inclusion required the presence of CD20+ lymphoplasmacytoid lymphoma involving the bone marrow, positive serum immunofixation for monoclonal IgM, and at least 1 of the consensus criteria for initiation of treatment.2 DRC consisted of six 21-day courses of dexamethasone 20 mg IV, followed by rituximab IV 375 mg/m2 and oral cyclophosphamide 100 mg/m2 twice daily (days 1 to 5). Patients without progressive disease were followed without treatment. Response was evaluated on an intention-to-treat basis per standard criteria.4 The cause of death was assessed prospectively by the treating physicians as either “WM-related” (ie, resulting from progressive disease, transformation to myelodysplastic syndrome [MDS] or diffuse large B-cell lymphoma [DLBCL], infections, or treatment-related complications), or “unrelated” (ie, died while WM was in remission, off treatment, resulting from causes such as stroke, myocardial infarction, or a second cancer, and without evidence of disease progression or relapse during this period). The characteristics of the patients, toxicity, and response have been published.3 On intent-to-treat, 83% achieved a response (complete remission: 7%, partial remission: 67%, minor remission: 9%); 9% had stable and 8% experienced progressive disease. Median progression-free survival (PFS) was 35 months (95% confidence interval, 22 to 48 months); disease progression at 3 years was 45%, and unrelated death, without disease progression, was 12% (Figure 1). In comparison, median PFS after single-agent rituximab was 23 months.5 Other rituximab-containing regimens (such as bortezomib, dexamethasone, rituximab) were also associated with a median PFS of around 3 years; however, these included an additional toxicity profile and shorter follow-up.6 Bendamustine with rituximab was superior to R-CHOP (PFS 69 vs 28 months with less toxicity),7 however the numbers at each group were small and the follow-up was short.
Time to event curves for patients in the phase 2 DRC study. (A) Overall survival (OS) according to age (≤65, 66 to 75, and 75 years). (B) WM-related and unrelated mortality. (C) OS per response category (including very good partial response [VGPR] category as per the revised response criteria published by Owen et al1 ). (D) Progression, and unrelated mortality without progression. CR, complete response; MR, minor response.
Time to event curves for patients in the phase 2 DRC study. (A) Overall survival (OS) according to age (≤65, 66 to 75, and 75 years). (B) WM-related and unrelated mortality. (C) OS per response category (including very good partial response [VGPR] category as per the revised response criteria published by Owen et al1 ). (D) Progression, and unrelated mortality without progression. CR, complete response; MR, minor response.
Forty patients (56%) received second-line therapy (Figure 2). Median time to next treatment was 51 months. Among patients who received second-line treatment, 28 (70%) were retreated with a rituximab-based regimen with high response rates (82% achieved at least a minor response); 12 patients (30%) were treated with alkylating agents, fludarabine, or bortezomib (67% achieved at least a minor response). Thus in patients who have achieved a relatively long-lasting response with initial rituximab-based therapy, a similar regimen may be considered as a reasonable option at relapse.
Outcomes of patients who participated in the phase 2 study of the combination of DRC in previously untreated patients with symptomatic WM. PD, progressive disease; SD, stable disease.
Outcomes of patients who participated in the phase 2 study of the combination of DRC in previously untreated patients with symptomatic WM. PD, progressive disease; SD, stable disease.
Thirty-five patients (49%) have died: 20 (57%) were WM-related, and in 15 (43%) death was unrelated to WM (related to solid tumors in 8, [lung in 4, bladder in 1, melanoma in 1, gastric in 1, and pancreatic in 1], heart disease in 4, stroke in 2, and pancreatitis in 1). Secondary MDS and transformation to DLBCL occur in ∼3% and ∼10% of WM patients, respectively.8-10 However, only 1 developed MDS after he had received fludarabine, and 2 patients (2.7%) developed DLBCL (1 after exposure only to DRC and the other after multiple treatments, including nucleosides). The rate of nonhematologic tumors was higher than MDS/DLBCL; whether the incidence of solid tumors is higher than expected for this elderly population and if these are related to WM or therapy requires further investigation. Median OS is 95 months (95% confidence interval, 87-103) and 8-year OS is 47%. Accounting for unrelated deaths as a competing event, WM-related death at 8 years was 32% and WM-unrelated was 21%. The 8-year and estimated 10-year OS related to WM was 64% and 53%, respectively. Thus we prospectively documented that unrelated mortality has significant impact on the survival of WM patients. The 8-year OS per the international prognostic scoring system for WM was 100%, 55%, and 27% for low, intermediate, or high-risk disease, respectively (P = .005).
In conclusion, PFS after DRC is about 3 years, whereas retreatment is feasible. The OS after primary DRC was about 8 years, affected by the risk status; however, second nonhematologic cancers were observed and about one-fifth of patients died of unrelated causes.
The online version of this article contains a data supplement.
Authorship
Contribution: E. Kastritis and M.A.D. collected and analyzed data, wrote the manuscript, and performed statistical analysis; and M.G., M.-C.K., M.R., E.H., A.S., P.R., E.M., S.D., K.T., P.T., A.V., E.V., E. Katodritou, D.G., and E.T. treated patients, collected and analyzed data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: M.A.D. received honoraria from Celgene, Janssen, and Onyx. E.T. received honoraria from Celgene and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Meletios A. Dimopoulos, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, School of Medicine, 80 Vas. Sofias Av, Athens, Greece, 11528; e-mail: mdimop@med.uoa.gr.

![Figure 1. Time to event curves for patients in the phase 2 DRC study. (A) Overall survival (OS) according to age (≤65, 66 to 75, and 75 years). (B) WM-related and unrelated mortality. (C) OS per response category (including very good partial response [VGPR] category as per the revised response criteria published by Owen et al1). (D) Progression, and unrelated mortality without progression. CR, complete response; MR, minor response.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/11/10.1182_blood-2015-05-647420/4/m_1392f1.jpeg?Expires=1769093618&Signature=S-7D3C50hrTfszUwnkzSgk~aJ0SQpRmfAuxyaPbuvce1TUCFej9HkDKNUyb2AHW~-uCf6ZwKy68MfKVxKzdhSyf687SFf-6Co11fJuoJQK1Uhc8KBaHWHJnDEwmetbjFW~rQiZ24j3v2dny3MXTMZAzhZeFsj9U65CYlrgKcyM2uYTxOrFc~O4E73F7O4eHoxCUBgpkk0UQ8lKJT1FVn~R-IQC1mrvdu~edYEcrBe0U7W2D75lMCcvTauiqx0ucbwUXVqoZISzhyguvDbwYmnjo8nBfqLYssVaId9WQWwHJQDQMsuRT0TtvHxnN61TPeqdesEiP-~oEQkj7K6t7c4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal