In this issue of Blood, Nickel et al show that long-chain polyphosphates (polyP) on the surface of secreted microvesicles (MVs) from prostate cancer cells activate coagulation factor XII (FXII), leading to thrombosis.1
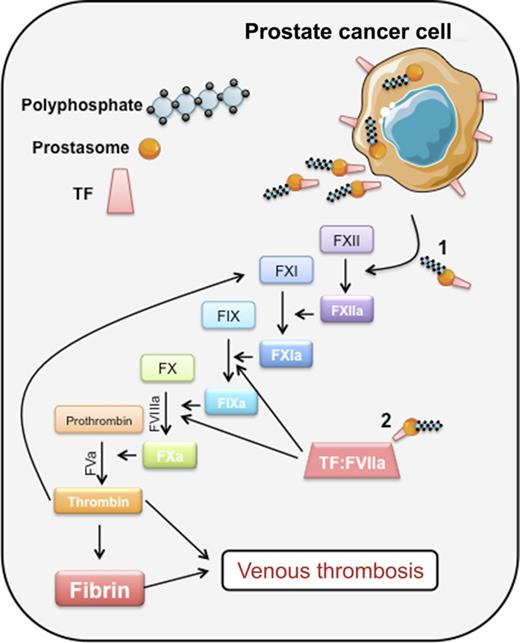
Prostate cancer cells synthesize and secrete prostasomes into body fluids. Prostasomes are prothrombotic by virtue of their expression of: (1) long-chain polyP, which can promote FXII auto-activation and initiation of thrombin via the intrinsic pathway; and (2) TF, which, in complex with factor VII(a), initiates coagulation through the extrinsic pathway. Professional illustration by Erica Sparkenbaugh.
Prostate cancer cells synthesize and secrete prostasomes into body fluids. Prostasomes are prothrombotic by virtue of their expression of: (1) long-chain polyP, which can promote FXII auto-activation and initiation of thrombin via the intrinsic pathway; and (2) TF, which, in complex with factor VII(a), initiates coagulation through the extrinsic pathway. Professional illustration by Erica Sparkenbaugh.
Cancer accounts for 20% to 30% of all cases of venous thromboembolism (VTE). The incidence of VTE in cancer is increasing, and it portends poorer survival than the absence of thrombosis. Since the association between cancer and thrombosis was first recognized in the early 19th century, numerous studies have addressed possible underlying pathophysiologic mechanisms. Candidate prothrombotic pathways have included proadhesive and procoagulant tumor cell-derived mucins, cysteine proteases with direct factor X activating activity, and tissue factor (TF), a transmembrane glycoprotein and the primary physiologic initiator of coagulation.2
More recently, research in this field has focused on MVs (or microparticles) released from tumor cells in the form of ectosomes and exosomes. Ectosomes are derived from the cell membrane and vary in size from 100 to 1000 nm. Exosomes, with a diameter in the 30 to 100 nm range, are released when cytoplasmic multivesicular bodies fuse with the plasma membrane.3 At least some MVs are endowed with prothrombotic cargo molecules derived from their cell of origin. The presence of these entities in the circulation potentially explains how thrombotic events occur at sites remote from the primary tumor or metastases. For example, TF has been identified in plasma samples on the surface of MVs derived from several tumor types, as well as from host cells such as monocytes. Despite compelling evidence that MV-associated TF can initiate thrombosis in mouse models of cancer,4 human studies evaluating whether the measurement of MV-associated TF is a clinically useful biomarker of thrombotic risk in cancer have produced mixed results.5
The past decade has witnessed a resurgent interest in the contact activation pathway of coagulation, composed of the zymogen FXII, high molecular weight kininogen, and pre-kallikrein. In vitro, deficiency of any of these proteins is associated with prolongation of the activated partial thromboplastin time, due to the inability to generate FXIIa, one of two known activators of factor XI (FXI) (see figure). Patients with a deficiency of any of the contact factors do not have a bleeding propensity and, unlike FXI-deficient subjects, are not known to be at lower risk of thrombosis. However, genetic or pharmacologic inhibition of FXII(a) is protective against thrombosis in mouse models of arterial and venous thrombosis. Importantly, treated animals do not manifest increased bleeding when hemostatically challenged.6 Because activation of the intrinsic pathway is triggered by contact-induced autoactivation of zymogen FXII, the question arises as to what anionic surfaces could function in this capacity in vivo. Misfolded proteins, high concentrations of free RNA or DNA, collagen, and sulfatides have all been implicated, but perhaps the most compelling candidates to date are long-chain inorganic polyPs, composed of ∼100 or more phosphates in a linear polymer. Long-chain polyPs are found in many bacteria. PolyP is also found in mammalian cell organelles, including lysosomes and dense granules. Platelet polyP, although abundant in dense granules, is considered to be medium chain length (60 to 100 phosphate molecules), which limits its ability to activate FXII, but does not interfere with the other procoagulant effects of polyP, including acceleration of factor V activation, promotion of thrombin activation of FXI, and inhibition of the TF pathway inhibitor.7
Although prostate cancer is not considered to be among the cancers with a high inherent risk of thrombosis, the mechanism of VTE associated with prostate cancer and mediated by tumor cell–derived prostasomes is the topic of the study by Nickel et al. Prostasomes are submicron vesicles (average diameter = 150 nm) that are secreted by prostatic epithelial cells into seminal fluid, where they participate in sperm motility.8 It has been known for many years that seminal fluid is procoagulant and profibrinolytic. Soluble hemostatic proteins that are relatively abundant in seminal fluid include factor VIII, prothrombin, urokinase-type plasminogen activator, and tissue-type plasminogen activator, whereas TF is present on prostasomes.9 Many prostatic cancer cells retain the capacity to secrete TF-bearing prostasomes, which can be detected in plasma and in other body fluids.10 Nickel et al examined the prothrombotic activities of secreted prostasomes derived from prostatic cancer cell lines and human seminal fluid. Long-chain polyP (200- to 1000-mers) was purified from these sources. The procoagulant activities of prostasomes were tested both by examining their effect in platelet-poor plasma, and by infusion into mice in a lethal pulmonary embolism model. Inhibition of FXII mitigated thrombin generation in plasma, and was protective against thrombosis without increased bleeding propensity in vivo. However, the procoagulant activity of prostasomes was also in part TF-dependent both in vitro and in vivo, raising the possibility that the role of FXII is to amplify TF-initiated coagulation, at least in this model, where a well-defined molecule is present to provide a surface for FXII auto-activation (see figure). Whether FXII activation initiates coagulation in vivo independently of TF and in the absence of exposure to an extracorporeal surface remains a topic for further study. Additional work is also needed to evaluate how commonly MV-borne polyP is present in the circulation of patients with other types of cancer. But these limitations do not mean that targeting FXII to limit thrombosis, with the added assurance of a wide therapeutic margin for bleeding, should not be pursued as a potential strategy in patients with thrombosis associated with cancer.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal