Key Points
Mouse inflammation models cause accumulation of B cells in the bone marrow within 12 hours and prior to peak emergency granulopoiesis.
Marrow B cells undergo spatial reorganization and are subjected to an altered cellular and secreted milieu.
Abstract
Systemic inflammation perturbs the bone marrow environment by evicting resident B cells and favoring granulopoiesis over lymphopoiesis. Despite these conditions, a subset of marrow B cell remains to become activated and produce potent acute immunoglobulin M (IgM) responses. This discrepancy is currently unresolved and a complete characterization of early perturbations in the B-cell niche has not been undertaken. Here, we show that within a few hours of challenging mice with adjuvant or cecal puncture, B cells accumulate in the bone marrow redistributed away from sinusoid vessels. This response correlates with enhanced sensitivity to CXC chemokine ligand 12 (CXCL12) but not CXCL13 or CC chemokine ligand 21. Concurrently, a number of B-cell survival and differentiation factors are elevated to produce a transiently supportive milieu. Disrupting homing dynamics with a CXC chemokine receptor 4 inhibitor reduced the formation of IgM-secreting cells. These data highlight the rapidity with which peripheral inflammation modifies the marrow compartment, and demonstrate that such modifications regulate acute IgM production within this organ. Furthermore, our study indicates that conversion to a state of emergency granulopoiesis is temporally delayed, allowing B cells opportunity to respond to antigen.
Introduction
For the majority of adult mouse and human B cells, development occurs in the bone marrow before final splenic maturation. It is well recognized that a variety of cellular and secreted factors within this organ collaborate to provide niches important in maintaining normal B-cell development. For example, parenchymal stromal cells have been shown to secrete IL-7, which is critical for the differentiation and proliferation of pre-B cells.1,2 Another group of stromal cells produces CXC chemokine ligand 12 (CXCL12), a chemokine regulating early pre-pro-B-cell generation as well as the movement and homing of all B-cell progenitors within the bone marrow.1,3 Recently, it has been demonstrated that reduced responsiveness of immature and transitional B cells to CXCL12, mediated by downregulation of the receptor CXC chemokine receptor 4 (CXCR4), attracts them to bone marrow sinusoids and eventually into circulation.4 The sinusoids themselves represent a niche supportive of B-cell survival and immature B cells load inside sinusoids before leaving for the periphery.5,6 These sinusoids, and adjacent architecture, support a sizable population of “recirculating” B cells composed of naive immunoglobulin D+ (IgD+) mature cells. The majority of this population are cells that passed through splenic maturation whereas roughly a third completed development within the bone marrow itself.7-9 Survival factors, such as macrophage migration inhibitory factor and B cell activating factor, are provided by the cohabitation of dendritic cells and likely a subset of DX5+Thy-1lo granulocytes.10-13 T cells are also found in close association.10
Although clearly a central lymphoid organ, various studies indicate that the bone marrow has a far more dynamic and multifunctional nature than typically appreciated. The bone marrow supports an important plasma cell niche, is a site of potent T-cell priming, and mature bone marrow B cells have been shown to respond within 4 days to Salmonella infection to produce bacterial specific IgM.7,14,15 Using either adjuvant or infection models, it has also been observed that lymphopoiesis is rapidly repressed and B cells make an exodus to the periphery mediated by reduced availability of CXCL12.16,17 This phenomenon is thought to make way for emergency granulopoiesis, a condition of elevated granulocyte maturation that occurs to replace innate immune cells, which may have been lost in the initial onslaught against a pathogen.18,19 However, this model does not explain how bone marrow B cells are able to produce antibody responses despite apparent instability of the population and their environment.
In the current study, we challenged mice with intraperitoneally injected incomplete Freund adjuvant (IFA), or by cecal puncture, and examined the very early influences of systemic inflammation on the bone marrow B-cell compartment. We show that, in the first hours following onset of inflammation, there is a large accumulation of mature B cells in the bone marrow. This response correlates with enhanced B-cell sensitivity to CXCL12 downstream of extrinsic interleukin 1 receptor (IL-1R) signaling. These effects are demonstrated to alter B-cell organization within the bone marrow itself; mature and immature B cells exhibit reduced localization to the sinusoids. Concurrently, a number of environmental modifications occur, including increases in both secreted cytokines and cellular components, indicating that acute inflammation transiently produces a bone marrow environment favorable for the maintenance and differentiation of mature B cells. Furthermore, we observe that disruption of this accumulation process with a CXCR4 inhibitor attenuates acute-phase IgM production.
Methods
Mice
C57BL/6 (CD45.2 and CD45.1), Rag1−/−, and Il1r1−/− mice were purchased from The Jackson Laboratory and housed under specific pathogen-free conditions in the animal facilities of Princess Margaret Cancer Centre, University Health Network (UHN). Il1r1−/− mice were bred in our animal facilities. Experimental mice were 7- to 16-week-old females and individual experiments always compared littermates. All experiments were approved by the UHN Animal Care Committee. IFA (Sigma) was prepared immediately prior to injection by vortexing IFA 1:1 with phosphate-buffered saline (PBS) for 30 minutes. Each mouse was injected intraperitoneally (i.p.) with 300 μL. Control mice were either injected with PBS or untreated, as indicated. Some mice simultaneously received lipopolysaccharide (LPS) at 0.5 mg/kg, or 1 mg/kg AMD3100 i.p. For splenectomy, mice were started on Baytril (Bayer) 1 week prior to surgery. Experiments were performed at least 2 weeks after surgery. FTY-720 was administered at 1 mg/kg. Cecal puncture was performed using a 23G needle to make a single puncture completely through the cecum and a small drop of feces extruded through the puncture. This is an adaptation from our previously described technique.20 Sham surgeries were conducted with the same pre- and postoperative procedures as treatment groups and the peritoneal cavity opened. In vivo sinusoidal labeling was accomplished by IV injection of 500 ng of rat anti-mouse B220 (RA3-6B2; eBioscience) or anti-Gr-1 (RB6-8C5; eBioscience) 5 minutes prior to euthanasia.
Cell isolation and flow cytometry
Single-cell bone marrow suspensions were made by flushing femurs and tibiae with PBS + 2% fetal calf serum (FCS). Spleen cells were isolated by mashing each spleen through a 40-μm cell strainer in PBS + 2% FCS. Blood was collected by cardiac puncture. All cell suspensions were treated with ACK buffer for red cell lysis. For flow cytometry, cell suspensions were stained: fluorescein isothiocyanate (FITC)–anti-FAS (2E7; BD Biosciences), FITC–anti-CD80 (16-10A1; BD Biosciences), FITC–anti-CD41 (MWReg30; BioLegend), FITC–anti-Gr-1 (RB6-8C5; eBioscience), phycoerythrin (PE)–anti-Ki67 (SolA15; eBioscience), PE–anti-CD86 (GL1; BioLegend), PE–anti-CD256 (A3D8; BioLegend), PE–anti-CD93 (AA4.1; BioLegend), PE–anti-CD11b (M1/70), biotin-conjugated peanut agglutinin (PNA; Sigma), biotin–anti-CD49b (DX5; BD Biosciences), biotin–anti-CD23 (B3B4; BioLegend), biotin–anti-CD69 (H1.2F3; BD Biosciences), streptavidin–peridinin chlorophyll (PERCP; BioLegend), biotin–anti-CD11c (N418; BioLegend), allophycocyanin (APC)–anti-CD19 (1D3; BioLegend), APC–anti-CXCR4 (2B11; eBioscience), APC–anti-CD21/35 (7E9; BioLegend), APC–anti-CD43 (S7; BioLegend), APC–anti-major histocompatibility complex II (MHCII) (M5/114.15.2; BioLegend), Alexa 647–anti-SiglecF (E50-2440; BD Pharmingen), PE-CY7–anti-IgM (B12.15F9; eBioscience), PE-CY7–anti-CD90.2 (53-2.1; eBioscience), eFluor450–anti-IgD (11-26; eBioscience), Pacific Blue–anti-CD45.2 (104; BioLegend). Dead cells were excluded with Zombie UV Fixable viability dye (BioLegend). The FOXP3/Transcription Factor Staining Buffer Set (eBioscience) was used for intracellular antigens. For cell cycle analysis, DNA was stained with 4,6 diamidino-2-phenylindole (DAPI; BioLegend). Flow cytometry was conducted using an LSRFortessa 5-laser (325; 405; 488; 561; 632) configuration (BD Biosciences).
Enumeration of total organ cell numbers
Immediately prior to analysis, isolated cell suspensions were diluted in Trypan Blue (Sigma) and live cells counted using a hemocytometer. To calculate the total bone marrow number, the percentage of flow cytometry gated live cells was multiplied by 10.6 because radiographic isotype distribution studies demonstrate that 1 mouse femur and tibia contain 9.4% of the total marrow.7,21
Chemotaxis assays
Total bone marrow was suspended in OPTI-MEM medium (Life Technologies), supplemented with 5% FCS (Life Technologies), and 5.5 × 10−5 M β-mercaptoethanol. Cells (1 × 106) were plated in the top chamber of a transwell with a 5-μm pore size (Costar). Media containing recombinant CXCL12 (100 ng/mL), CXCL13 (500 ng/mL), or CC chemokine ligand 21 (CCL21) (250 ng/mL; R&D Systems) was added to the bottom chamber. After incubating for 3 hours at 37°C, 5% CO2, cells in the bottom chamber were counted and analyzed by flow cytometry. Normalized percent chemotaxis was calculated by comparing bottom chamber cell population counts with input numbers. This was done for both control and IFA samples to normalize for differences in the input population composition.
Adoptive transfer and mixed bone marrow chimeras
IgD+ splenic cells were sorted by fluorescence-activated cell sorter (FACS) and 5 × 106 of cells were injected IV into recipients immediately prior to IFA challenge. CD45.2 IL-1R–deficient CD19+ splenic cells were isolated from naive mice by magnetic-activated cell sorting (MACS; Stem Cell Technologies) and 5 × 106 of cells were injected IV into CD45.1 wild-type recipients. To make mixed bone marrow chimeras, CD45.1 wild-type mice were irradiated with 950 rad and IV injected with 1 × 106 each of CD45.2 Il1r1−/− and CD45.1 wild-type total bone marrow. Mice were maintained on Baytril (Bayer) for 1 week prior and 2 weeks postirradiation. We allowed 6 weeks for reconstitution before challenging with IFA.
Enzyme-linked immunospot
Ninety-six-well polyvinylidene difluoride (PVDF) filter plates (Millipore) were prewetted with 70% ethanol and then coated with 10 μg/mL polyclonal anti-IgM (The Jackson Laboratory) in PBS. After blocking with OPTI-MEM medium containing 5% FCS, 5 × 105 total bone marrow cells were plated and incubated overnight at 37°C before detecting with polyclonal horseradish peroxidase (HRP)–anti-IgM (Sigma). Spots were developed with 3-amino-9-ethyl-carbazole (AEC; Sigma) and counted using ImmunoSpot analysis software (Cellular Technology Limited).
Cytokine analysis
To collect bone marrow extracellular fluid, 1 tibia and femur were centrifuged in a microfuge tube at 3000 rcf. The resulting pellet was resuspended in 100 μL of PBS and centrifuged once again. These supernatants were analyzed in a multiplex cytokine array (Eve Technologies). CXCL12 was enumerated by enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Statistical analysis
All statistical analysis was performed using Prism (GraphPad Software). For most data, statistical significance was determined using a Student t test. Figures 1C-D, 3E, and 7C were analyzed using a 1-way analysis of variance (ANOVA) and Dunnett or Bonferroni post test. ★P ≤ .05; ★★P ≤ .01; ★★★P ≤ .001.
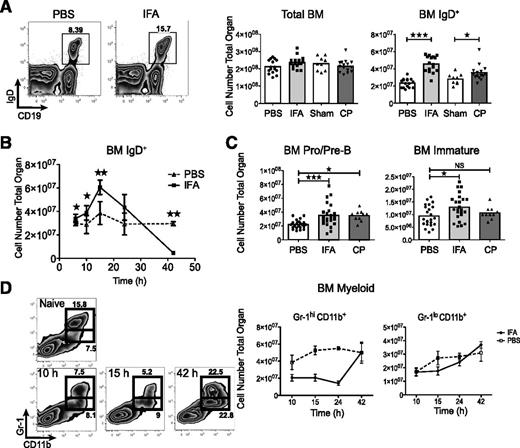
During systemic inflammation B cells accumulate in the bone marrow prior to full onset of emergency granulopoiesis. (A) Representative flow plots and IgD+ bone marrow cell numbers following IFA and lethal dose90 cecal puncture as compared with PBS or sham mice. Data are pooled from 3 independent experiments and each point indicates an individual mouse. The bar represents the population mean. (B) IgD+ cell numbers plotted over time. The experiment was repeated twice, n = 5 at each time point and error bars are SD. (C) Immature B cells (CD19+IgD−IgM+CD93+), and pro-/pre-B (CD19+IgD−IgM−CD93+) were enumerated. Data are pooled from 4 (IFA) or 2 independent experiments (cecal puncture). (D). Granulocyte progenitors and mature neutrophils were followed in a time course. The experiment was repeated twice, n = 5 at each time point and error bars are SD. ★P ≤ .05; ★★P ≤ .01; ★★★P ≤ .001 by unpaired Student t test, or 1-way ANOVA and Dunnett post test.
During systemic inflammation B cells accumulate in the bone marrow prior to full onset of emergency granulopoiesis. (A) Representative flow plots and IgD+ bone marrow cell numbers following IFA and lethal dose90 cecal puncture as compared with PBS or sham mice. Data are pooled from 3 independent experiments and each point indicates an individual mouse. The bar represents the population mean. (B) IgD+ cell numbers plotted over time. The experiment was repeated twice, n = 5 at each time point and error bars are SD. (C) Immature B cells (CD19+IgD−IgM+CD93+), and pro-/pre-B (CD19+IgD−IgM−CD93+) were enumerated. Data are pooled from 4 (IFA) or 2 independent experiments (cecal puncture). (D). Granulocyte progenitors and mature neutrophils were followed in a time course. The experiment was repeated twice, n = 5 at each time point and error bars are SD. ★P ≤ .05; ★★P ≤ .01; ★★★P ≤ .001 by unpaired Student t test, or 1-way ANOVA and Dunnett post test.
Results
B cells rapidly accumulate in the bone marrow during acute inflammation
As bacterial sepsis can quickly cause severe morbidity, we were prompted to assess the speed with which bone marrow B cells are affected by systemic inflammation.22 Within 12 hours we observed a near doubling of IgD+ B cells in the bone marrow of mice challenged i.p. with IFA (Figure 1A).17 This expansion was also evident 12 hours following cecal puncture (Figure 1A). Detailed analysis after IFA administration demonstrated that B cells began expanding in the first few hours, peaked around 15 hours, and declined to below normal levels by 42 hours (Figure 1B). We also observed a slight, but statistically significant, increase in pro- and pre-B cells 12 hours following both IFA administration and cecal puncture. A very modest expansion of immature B cells was evident after IFA treatment, but not cecal puncture (Figure 1C).
Given the known correlation between emergency granulopoiesis and B-cell populations, we examined the frequency of myeloid subsets during the same time frame explored for B cells. Using the markers Gr-1 and CD11b, we noted that although mature cells (Gr-1hiCD11b+) rapidly declined, there was no change to the progenitor population (Gr-1loCD11b+) until 42 hours after IFA treatment. This coincided with replenishment of Gr-1hiCD11b+ cells (Figure 1D).18
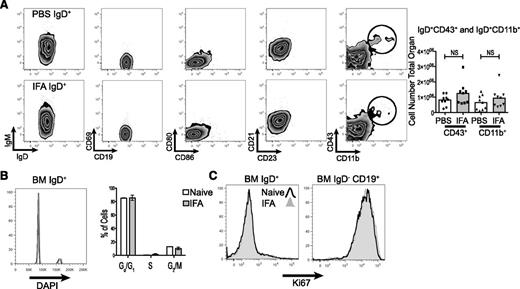
A detailed flow cytometric examination of surface markers confirmed that the excess B cells were largely mature, naive, and of the B-2 lineage. Gating on the IgD+ population revealed that these cells maintained normal expression of IgM, CD21, CD23, and were not positive for the activation markers CD69, CD80, or CD86. There was also no change in the absolute number of IgD+CD43+ or IgD+CD11b+ cells (Figure 2A). To determine whether this expanded B-cell population was the result of local proliferation, we examined proliferative activity by cell cycle analysis and Ki67 staining. Neither measure indicated a difference from control (Figure 2B-C).
The expanded IgD+ population is composed mostly of naive, nonproliferating, B-2 lineage cells. (A) The cellular phenotype of bone marrow IgD+ cells was determined by flow cytometry 12 hours following IFA injection. Representative flow plots are shown. CD11b+ and CD43+ cells were enumerated across 2 independent experiments. (B) Fixed and permeablized bone marrow cells were stained with DAPI and B-cell makers to analyze cell cycle. Shown is a representative histogram comparing naive and IFA mice at 12 hours. The percentage of IgD+ cells in each cell cycle phase was tabulated and is graphed, n = 5 in each group and error bars are SEM. (C) Bone marrow populations were stained for intracellular Ki67. A representative histogram for IgD+ and progenitor populations is shown. The experiment was repeated twice.
The expanded IgD+ population is composed mostly of naive, nonproliferating, B-2 lineage cells. (A) The cellular phenotype of bone marrow IgD+ cells was determined by flow cytometry 12 hours following IFA injection. Representative flow plots are shown. CD11b+ and CD43+ cells were enumerated across 2 independent experiments. (B) Fixed and permeablized bone marrow cells were stained with DAPI and B-cell makers to analyze cell cycle. Shown is a representative histogram comparing naive and IFA mice at 12 hours. The percentage of IgD+ cells in each cell cycle phase was tabulated and is graphed, n = 5 in each group and error bars are SEM. (C) Bone marrow populations were stained for intracellular Ki67. A representative histogram for IgD+ and progenitor populations is shown. The experiment was repeated twice.
Expanded IgD+ cells are derived from the blood recirculating lymphocyte pool
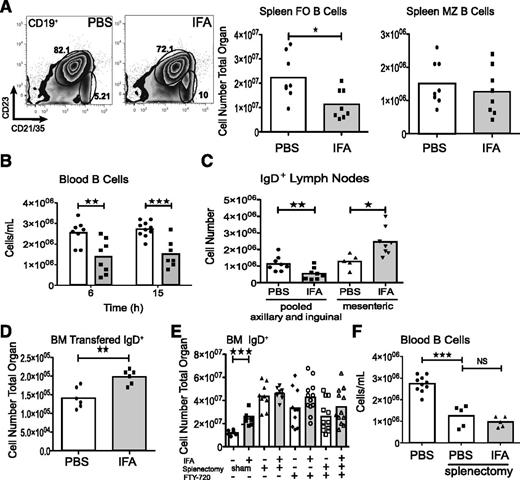
To further examine B-cell dynamics after IFA injection, we quantified B-cell populations in the peripheral blood and spleen. A sizable reduction of the splenic follicular B-cell compartment (CD19+CD23+CD21/35+) was evident, but the number of marginal zone B cells (CD19+CD23lo/−CD21hi) remained unchanged (Figure 3A). Similarly, within 6 hours there was a decrease in peripheral blood B cells (Figure 3B). The axillary and inguinal lymph nodes also exhibited fewer IgD+ cells but an expansion of this population was observed in the draining mesenteric lymph node (Figure 3C). As we suspected, these changes reflected movement of peripheral B cells into the bone marrow we adoptively transferred labeled IgD+ cells from naive spleens immediately prior to treating recipients with IFA (Figure 3D).
Accumulating B cells are derived from the recirculating lymphocyte pool. (A) Splenic follicular (CD19+CD23+CD21+) and marginal zone (CD19+CD23loCD21hi) B-cell populations were quantified following IFA treatment. Data are pooled from 2 experiments. (B) Blood B cells were enumerated by flow cytometry. Data are pooled from 2 independent experiments. (C) Single axillary and inguinal lymph nodes were pooled and analyzed together with mesenteric lymph nodes by flow cytometry. Data are pooled from 2 independent experiments. (D) Splenic IgD+ cells were sorted by FACS and labeled with Cell Tracker Orange before IV injection. Data are from 2 experiments. (E) Two weeks after splenectomy mice were challenged with IFA for bone marrow analysis. In additional experiments intact or splenectomized mice were treated with FTY-720 immediately prior to IFA. Data are from 2 or 3 experiments. (F) B-cell blood counts are compared between the data from (B) and splenectomized mice. Data are pooled from 2 experiments.
Accumulating B cells are derived from the recirculating lymphocyte pool. (A) Splenic follicular (CD19+CD23+CD21+) and marginal zone (CD19+CD23loCD21hi) B-cell populations were quantified following IFA treatment. Data are pooled from 2 experiments. (B) Blood B cells were enumerated by flow cytometry. Data are pooled from 2 independent experiments. (C) Single axillary and inguinal lymph nodes were pooled and analyzed together with mesenteric lymph nodes by flow cytometry. Data are pooled from 2 independent experiments. (D) Splenic IgD+ cells were sorted by FACS and labeled with Cell Tracker Orange before IV injection. Data are from 2 experiments. (E) Two weeks after splenectomy mice were challenged with IFA for bone marrow analysis. In additional experiments intact or splenectomized mice were treated with FTY-720 immediately prior to IFA. Data are from 2 or 3 experiments. (F) B-cell blood counts are compared between the data from (B) and splenectomized mice. Data are pooled from 2 experiments.
To confirm splenic contribution, we challenged splenectomized mice with IFA. In agreement with previous studies, splenectomy expanded the bone marrow B-cell compartment.8,23 However, we found that these mice had comparatively unchanged numbers of IgD+ cells 12 hours following IFA injection (Figure 3E). Although this implicates splenic B cells as source of cell accumulation in the bone marrow, we noted that treatment with FTY-720, a S1PR agonist which sequesters B cells in secondary lymphoid organs, yielded similar attenuation of IgD+ expansion (Figure 3E).24 Furthermore, splenectomy itself induced lymphopenia (Figure 3F).
Inflammation enhances B-cell sensitivity to CXCL12
We hypothesized that B-cell mobilization would be dependent on chemokine activity. Various chemokines have a critical role in B-cell trafficking, both in inflammation and in steady state.24,25 The CXCR4-CXCL12 axis is particularly important in controlling B-cell homing to and from the bone marrow.4 It was previously demonstrated that CXCL12 message within the bone marrow decreases 3 days after IFA treatment and this contributed to the loss of bone marrow B cells.17,18 We could find no indication of reduced CXCL12 12 hours after IFA (Figure 4A). However, various stimuli (including inflammatory) are capable of modifying the sensitivity of chemokine receptors for their ligand.26 To see if a sensitization effect occurred after acute inflammation, we conducted chemotaxis assays comparing B cells from control animals to those that had undergone cecal puncture or were injected with IFA. All bone marrow B cells from treated animals had greater chemotactic, but not chemokinetic, efficiency toward CXCL12 than cells from control mice (Figure 4B-D). To determine whether enhanced chemotaxis occurred with other chemokines, we repeated the experiments to include CXCL13 and CCL21. In contrast to CXCL12, there was no difference in chemotaxis (Figure 4E). Furthermore, injection of the CXCR4-specific inhibitor, AMD3100, was sufficient to rapidly counteract IgD+ cell accumulation and mobilize these cells from the bone marrow (Figure 4F).
Inflammation enhances B-cell sensitivity to CXCL12. (A) CXCL12 was measured in bone marrow extracellular fluid from 1 femur and tibia. Data are pooled from 3 experiments. (B-C) Twelve hours after challenging mice with IFA or cecal puncture, total bone marrow pooled from 3 mice was plated in a transwell to measure migration toward CXCL12. Data are representative of 3 independent experiments. (D) Chemokinetic potential was examined by adding CXCL12 in equal concentrations to both the transwell top and bottom chambers. Graph is representative of 2 independent experiments. (E) Chemotaxis assays for CXCL13 and CCL21 as compared with CXCL12. Each point represents the mean fold change in chemotaxis for an independent experiment. (F) IFA-treated mice were injected IP with 1 mg/kg AMD3100 1 hour prior to analysis. Data are pooled from 2 experiments. (G-H) B-cell expression level of CXCR4 was determined by flow cytometry measurement of median fluorescence intensity. (G) Error bars indicate standard error of the mean (SEM) and graph is a representative experiment, where n = 5. (H) Each point and connected line is the median fluorescence intensity (MFI) for a single experiment (n = 3-5 per experiment). (I) Cells were cultured for 1 hour in media containing 2.5 mM methyl-β-cyclodextran. Shown is a representative experiment.
Inflammation enhances B-cell sensitivity to CXCL12. (A) CXCL12 was measured in bone marrow extracellular fluid from 1 femur and tibia. Data are pooled from 3 experiments. (B-C) Twelve hours after challenging mice with IFA or cecal puncture, total bone marrow pooled from 3 mice was plated in a transwell to measure migration toward CXCL12. Data are representative of 3 independent experiments. (D) Chemokinetic potential was examined by adding CXCL12 in equal concentrations to both the transwell top and bottom chambers. Graph is representative of 2 independent experiments. (E) Chemotaxis assays for CXCL13 and CCL21 as compared with CXCL12. Each point represents the mean fold change in chemotaxis for an independent experiment. (F) IFA-treated mice were injected IP with 1 mg/kg AMD3100 1 hour prior to analysis. Data are pooled from 2 experiments. (G-H) B-cell expression level of CXCR4 was determined by flow cytometry measurement of median fluorescence intensity. (G) Error bars indicate standard error of the mean (SEM) and graph is a representative experiment, where n = 5. (H) Each point and connected line is the median fluorescence intensity (MFI) for a single experiment (n = 3-5 per experiment). (I) Cells were cultured for 1 hour in media containing 2.5 mM methyl-β-cyclodextran. Shown is a representative experiment.
Given the apparent specificity of IFA in enhancing CXCL12-driven chemotaxis, we assayed CXCR4 expression on B cells after IFA treatment. Flow cytometric analysis indicated normal surface levels on IgD+ cells and a reduction on progenitors (Figure 4G-H). As increased CXCR4 function and ligand-induced internalization could produce surface downregulation, we compared cells assayed directly ex vivo and those rested 1 hour in media containing 5% bovine serum albumin (BSA). In both cases surface and intracellular CXCR4 levels remained unchanged with IFA (Figure 4H). Previously, it has been shown that cells can be primed to have enhanced responses to CXCL12 gradients by recruitment of CXCR4 into cholesterol-rich membrane lipid rafts.27 To determine whether chemotactic enhancement could be suppressed by cholesterol depletion, we incubated cells 1 hour with the cholesterol chelator methyl-β-cyclodextran. This treatment abrogated IFA responses (Figure 4I).
B-cell extrinsic IL-1R signaling is upstream of enhanced CXCL12 sensitivity and IgD+ bone marrow accumulation
To determine which of the inflammatory pathways induced by peritoneal IFA injection are responsible for enhancing CXCL12-mediated chemotaxis, we analyzed Il1r1−/− mice for B-cell accumulation. These mice, while immunocompromised, as shown by reduced survival during sepsis, react at least partially to IFA challenge.28 Granulocyte colony-stimulating factor (G-CSF), which is rapidly and copiously produced in this model, appears to reach near wild-type levels in the serum and there were expected levels of granulocyte mobilization (data not shown). As shown in Figure 5A, 12 hours following IFA injection there was no significant accumulation of IgD+ cells in the bone marrow of Il1r1−/− mice. Moreover, B cells migrated with equal efficiency in CXCL12 chemotaxis assays comparing control and treated knockout animals (Figure 5B). Several reports indicate that B cells can express IL-1 type I receptors, especially under inflammatory conditions, so we took a 2-pronged approach to determine whether intrinsic IL-1 signaling modified CXCL12 responses.29,30 First, we generated mixed bone marrow chimeras by injection of an equal ratio of CD45.2 Il1r1−/− and CD45.1 wild-type bone marrow cells into CD45.1 lethally irradiated recipients. After allowing 6 weeks for reconstitution, we challenged with IFA and plated pooled total bone marrow in a CXCL12 chemotaxis assay as before. Enumerating the response of CD45.1 and CD45.2 IgD+ cells individually, we note that in 5 of 6 IFA wells there was an increase in chemotaxis as compared with wells from control mice. Importantly, in these 6 wells, there was no difference in the fold change (IFA/naive) in chemotaxis when comparing CD45.1 to CD45.2 cells (Figure 5C). In a parallel approach, we adoptively transferred 5 × 106 CD19+ cells from naive Il1r1−/− CD45.2 mice into wild-type CD45.1 recipients immediately preceding IFA injection. Twelve hours later, the absolute number of donor IL-1R–deficient B cells was significantly increased in recipient bone marrow (Figure 5D).
IL-1R is required for B-cell accumulation and CXCL12 responses. (A) Il1r1−/− mice were injected with IFA and analyzed for accumulation of bone marrow IgD+ cells. Data are pooled from 3 experiments. (B) Total bone marrow from 3 Il1r1−/− mice was plated in CXCL12 chemotaxis assay. Data are representative of 3 independent experiments. (C) A mixed chimera was made with wild-type and Il1r1−/− bone marrow. After reconstitution, mice were challenged with IFA and pooled bone marrow plated in a CXCL12 chemotaxis assay. The figure shows the fold change in chemotaxis of IFA over naive for both CD45.1 and CD45.2 cells. Data are from a single experiment with 6 control and 5 IFA-treated mice. (D) CD45.2 IL-1R–deficient B splenic B cells were transferred into CD45.1 recipients immediately prior to administration of IFA. Transferred cells were enumerated 12 hours later. Data are pooled from 2 experiments.
IL-1R is required for B-cell accumulation and CXCL12 responses. (A) Il1r1−/− mice were injected with IFA and analyzed for accumulation of bone marrow IgD+ cells. Data are pooled from 3 experiments. (B) Total bone marrow from 3 Il1r1−/− mice was plated in CXCL12 chemotaxis assay. Data are representative of 3 independent experiments. (C) A mixed chimera was made with wild-type and Il1r1−/− bone marrow. After reconstitution, mice were challenged with IFA and pooled bone marrow plated in a CXCL12 chemotaxis assay. The figure shows the fold change in chemotaxis of IFA over naive for both CD45.1 and CD45.2 cells. Data are from a single experiment with 6 control and 5 IFA-treated mice. (D) CD45.2 IL-1R–deficient B splenic B cells were transferred into CD45.1 recipients immediately prior to administration of IFA. Transferred cells were enumerated 12 hours later. Data are pooled from 2 experiments.
IFA alters B-cell localization as well as the marrow milieu
Given our observation that B cells demonstrate increased migratory response to CXCL12, we next tested whether inflammation changed the localization of B cells within the marrow itself. To do so, we administered an anti-B220 PE-conjugated antibody IV 5 minutes prior to euthanasia. By virtue of PE’s large molecular weight, this technique allows flow cytometric discrimination between cells within the bone marrow sinusoids and parenchyma.5 As seen in Figure 6A, this in vivo labeling revealed a dramatic reduction in not only the number of IgD+, but also immature B cells in the sinusoids 12 hours after IFA treatment. Conversely, there was an expansion of the number of sinusoidal Gr-1+ cells when labeling was performed with anti-Gr-1 PE (Figure 6A). When the procedure was repeated in splenectomized mice, there was a similar reduction of sinusoidal B cells despite the relatively stable number of total marrow IgD+ (Figure 6B). Taken together, these data suggest that increased CXCL12 responsiveness of B cells mediates enhanced retention within the bone marrow, which accounts for our observations of reduced splenic and blood B-cell numbers during the first 24 hours.
IFA causes reorganization of the bone marrow B-cell environment. (A) The number of sinusoidal B cells and granulocytes was determined by in vivo labeling. Five hundred nanograms of αB220-PE or αLy6G-PE was injected IV 5 minutes prior to euthanizing the mice. Representative flow panels display sinusoidal frequency within gated IgD+ or immature (IgD-IgM+CD19+). Graphed data are pooled from 3 experiments for B cells or a representative experiment for granulocytes. (B) In vivo sinusoid labeling was performed on splenectomized mice. Shown is a representative experiment. (C) Bone marrow from IFA or untreated Rag1−/− mice was plated in equal ratios with CD19+ naive splenocytes for 18 hours with 10 μg/mL LPS. Data are representative of 3 independent experiments. Error bars are SEM. (D) Cytokine levels in the extracellular fluid of the bone marrow were examined with multiplex ELISA. Data are pooled from 2 experiments. The thresholds of detection are: IL-5, 0.7 pg/mL; IL-6, 1.8 pg/mL; IL-7, 0.9 pg/mL; CCL3, 8.3 pg/mL; IL-13, 6.3; Eotaxin, 4.4 pg/mL. (E) Thy1loDX5+ bone marrow cells were enumerated by flow cytometry. Representative flow plots are shown and graphed data are pooled from 4 experiments. (F) April expression was detected by intracellular flow cytometry. (G) CD11c+MHCIIhi, SigLecF+Gr-1loCD11b+SSChi and CD41+SSChiFSChipolyploid (n > 2) cells were enumerated by flow cytometry. Data are pooled from 2 experiments.
IFA causes reorganization of the bone marrow B-cell environment. (A) The number of sinusoidal B cells and granulocytes was determined by in vivo labeling. Five hundred nanograms of αB220-PE or αLy6G-PE was injected IV 5 minutes prior to euthanizing the mice. Representative flow panels display sinusoidal frequency within gated IgD+ or immature (IgD-IgM+CD19+). Graphed data are pooled from 3 experiments for B cells or a representative experiment for granulocytes. (B) In vivo sinusoid labeling was performed on splenectomized mice. Shown is a representative experiment. (C) Bone marrow from IFA or untreated Rag1−/− mice was plated in equal ratios with CD19+ naive splenocytes for 18 hours with 10 μg/mL LPS. Data are representative of 3 independent experiments. Error bars are SEM. (D) Cytokine levels in the extracellular fluid of the bone marrow were examined with multiplex ELISA. Data are pooled from 2 experiments. The thresholds of detection are: IL-5, 0.7 pg/mL; IL-6, 1.8 pg/mL; IL-7, 0.9 pg/mL; CCL3, 8.3 pg/mL; IL-13, 6.3; Eotaxin, 4.4 pg/mL. (E) Thy1loDX5+ bone marrow cells were enumerated by flow cytometry. Representative flow plots are shown and graphed data are pooled from 4 experiments. (F) April expression was detected by intracellular flow cytometry. (G) CD11c+MHCIIhi, SigLecF+Gr-1loCD11b+SSChi and CD41+SSChiFSChipolyploid (n > 2) cells were enumerated by flow cytometry. Data are pooled from 2 experiments.
Because the B cells themselves were reorganized into an abnormal distribution, we reasoned that there would be other marrow adaptations capable of influencing B-cell development or function. To address this hypothesis, we cocultured naive CD19+ splenocytes with equal ratios of total marrow from either IFA- or PBS-treated Rag1−/− mice in LPS-containing media. After 18 hours of culture, there was significantly higher representation of IgD+ cells in cultures initiated with inflammatory bone marrow (Figure 6C). To ascertain whether these in vitro findings were reflective of the in vivo environment, we analyzed bone marrow extracellular fluid for cytokine levels by multiplex ELISA, and potential expansion of cell populations known to be supportive of B-cell maintenance by flow cytometry. We observed significant increases in the levels of IL-5, IL-6, and IL-13, as well as a reduction in IL-7 (Figure 6D). All of these molecules have been implicated in influencing B-cell differentiation and antibody production.2,31-34 Eotaxin and CCL3/MIP-1α (macrophage inflammatory protein 1α) were also increased. We also noted marked expansion of the bone marrow DX5+Thy1loFcεRI+ side-scattered light (SSC)loT cell receptor (TCR)− population (Figure 6E). These cells have been previously presumed to be basophils and were shown to promote B-cell survival after IgM crosslinking.11,13,35 We further these findings by showing that this population is APRIL+, a factor linked to B-cell and plasma cell antibody induction (Figure 6F).36 There was also an expansion of CD11c+MHC-IIhi dendritic cells but not SigLecF+Gr-1loCD11b+SSChi eosinophils or CD41+SSChiforward-scattered light (FSC)hi polyploid megakaryocytes. These phenotypes have been variously linked to B-cell or plasma cell function in the bone marrow (Figure 6G).10,37,38
The bone marrow’s early response to peripheral inflammation shapes B-cell effector function
The observation that IFA quickly reorganized the bone marrow environment prompted us to examine whether these changes could direct subsequent B-cell activity. We began to address this question by determining how quickly IgM production could be induced following IFA administration. Enzyme-linked immunospot (ELISPOT) analysis performed on total splenocytes or bone marrow indicated 12 hours was sufficient to induce IgM secretion. Interestingly, the bone marrow compartment generated a response of similar magnitude to the spleen (Figure 7A).
Rapid formation of IgM-secreting cells is coupled to CXCR4-dependent localization in the bone marrow. (A) Twelve hours following IFA injection total bone marrow or spleen was assayed for IgM secretion by ELISPOT. Data are pooled from 2 experiments. (B) Four hours after IFA + LPS treatment, mice were injected i.p. with 1 mg/kg AMD3100. On day 2, PNA+FAS+ bone marrow B cells were enumerated by flow cytometry. Data are pooled from 2 experiments. (C) Total bone marrow from mice treated with AMD3100 as in panel B was plated in an IgM ELISPOT. Data are pooled from 3 experiments and significance determined by 1-way ANOVA and Dunnett post test (left panel). The mean spot count for 5 independent experiments is graphed (right panel) comparing IFA-treated mice with IFA + AMD3100. Each point and connecting line represents an individual experiment. (D) Spleens from the experiments shown in panel C, as well as IL-1R−/− mice were plated in an IgM ELISPOT.
Rapid formation of IgM-secreting cells is coupled to CXCR4-dependent localization in the bone marrow. (A) Twelve hours following IFA injection total bone marrow or spleen was assayed for IgM secretion by ELISPOT. Data are pooled from 2 experiments. (B) Four hours after IFA + LPS treatment, mice were injected i.p. with 1 mg/kg AMD3100. On day 2, PNA+FAS+ bone marrow B cells were enumerated by flow cytometry. Data are pooled from 2 experiments. (C) Total bone marrow from mice treated with AMD3100 as in panel B was plated in an IgM ELISPOT. Data are pooled from 3 experiments and significance determined by 1-way ANOVA and Dunnett post test (left panel). The mean spot count for 5 independent experiments is graphed (right panel) comparing IFA-treated mice with IFA + AMD3100. Each point and connecting line represents an individual experiment. (D) Spleens from the experiments shown in panel C, as well as IL-1R−/− mice were plated in an IgM ELISPOT.
To see if the early reorganization of B cells during acute inflammation contributed to the marrow’s capability to produce IgM, we analyzed Il1r1−/− mice 2 days after challenge with IFA + LPS. As measured by ELISPOT, there appeared to be fewer IgM-secreting cells (data not shown). However, a defect was also apparent in the spleen, indicating other mechanisms related to IL-1 could be at play (Figure 7D). Thus, we injected AMD3100 4 hours after challenging with IFA + LPS. AMD3100s interference with CXCR4 potently mobilizes B cells from the bone marrow so we reasoned that interrupting accumulation of B cells at an early time point would disrupt interactions necessary for subsequent activation and antibody production.39 Following AMD3100 treatment, there was an apparent reduction in the number of FAS+PNA+ B cells, as well as an attenuated IgM response in the bone marrow, but not the spleen (Figure 7B-D).
Discussion
In mice and humans, bone marrow is an organ of exceptional immunologic importance. Chiefly, it constitutes an adult animal’s primary source of newly generated hematopoietic lineage cells. However, a growing body of evidence indicates that bone marrow also maintains a high level of immune responsiveness and demonstrates characteristics of a secondary lymphoid organ.7,19,40 The marrow’s highly vascularized architecture means that within minutes of IV injection, blood-borne antigen is readily detectable; antigen-presenting cell uptake and cross-presentation to T cells follows within 3 hours.3,41 Several studies have also demonstrated active transport of antigen into the bone marrow environment by neutrophils and dendritic cells.42,43 Moreover, this organ provides a major niche for long-lived plasma cells and, when total volume is considered, houses approximately as many T cells as the spleen.14,15 B cells cannot be ignored either, as experiments utilizing a Salmonella infection model demonstrated that a portion of marrow-resident mature recirculating B-2 cells quickly became activated to produce bacterial-specific IgM.7
Another notable characteristic of the bone marrow environment is its tremendously dynamic nature. In response to a varied array of systemic inflammatory insults, changes in immune cell composition as well as availability of cytokines and chemokines are rapidly evident.17-19 One rationale for these alterations is to give myelopoiesis priority over lymphopoiesis; a hypothesis aptly demonstrated by the massive replacement of B-cell progenitors with myeloid precursors as early as 3 days after onset of inflammation.18,19 Although this model makes immunologic sense, as the need to replenish granulocytes during acute infection is obvious, it does not account for how subsets of B cells can remain to produce rapid antibody responses in an apparently hostile environment. Interestingly, our experiments reveal that B-cell production of IgM as well as potentially supportive environmental alterations, including increased IL-5, IL-6, IL-13, and expansion of APRIL+DX5+Thy1lo and CD11c+MHCIIhi cells, occurs prior to peak granulocyte production. We show that B cells themselves are redistributed within the marrow with fewer mature and immature B cells in the sinusoids. Previous studies suggest that the sinusoidal niche is of importance in dictating B-cell fate. In cannabinoid receptor2 knockout mice, which have a defect in the ability to maintain B cells in the sinusoids, splenic B cells demonstrate reduced λ light chain usage.5 Moreover, experiments using a transgenic B-cell receptor (BCR) system indicate that autoreactive B cells are restricted to the parenchyma and unable to migrate to the sinusoids; however, receptor edited B cells were found in the sinusoids at normal frequency.44 In our experiments, we noted an increase in Gr-1+ sinusoidal cells after IFA treatment. Conceivably, an influx of granulocytes to this location could heighten competition with B cells; the segregation of these 2 cell types seen during inflammation may act to prevent this outcome, but also alter B-cell maturation. In essence, as B-cell mobilization has been hypothesized to clear niche space for emergency granulopoiesis so too could initial granulocyte mobilization reduce competition for B cells.18 Accumulating B cells could then freely respond to blood-borne antigen before granulopoiesis gains priority.
The protective value of acute antibody production should not be overlooked, especially during sepsis. Studies using cecal puncture and ligation models have demonstrated that B cells and IgM bestow a clear survival benefit during the first 2 to 4 days of sepsis.45,46 A significant bone marrow contribution is supported by data presented here, which indicate near parity, in terms of the number of IgM-secreting cells, with the spleen. This is seen as early as the first 12 hours and sustained until at least day 2. Early IgM production may also have important implications for long-term antibody protection during infection. Mice defective in their ability to secrete IgM produced less IgG and had stalled affinity maturation during primary immunization. This could be mediated through IgM’s ability to activate compliment, or perhaps oligomerize antigen.47 A role for the bone marrow in this pathway could be to compel B cells to produce only IgM. This environment provides insufficient signals to induce activation-induced cytidine deaminase expression and thus no opportunity for class switch recombination.7 Our experiments support a view that the bone marrow organ, at least immediately following infection, is an excellent place for cells to encounter antigen and differentiate into an IgM-secreting cell. Our study bridges previously conflicting notions of the bone marrow during inflammation: an environment transforming to support emergency granulopoiesis and one ideal for launching adaptive immunity. By demonstrating that an influx of B cells, and the environmental reorganization necessary to support their effector activity, occurs prior to peak granulopoiesis, we provide evidence that these events are temporally structured to provide maximal immunologic advantage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the University Health Network (UHN) Animal Care Facility, and particularly Sadiya Yousef and Amanda Healy for lending their expert surgical skills. They also thank Betty Fan and Francis Tong for FACS assistance.
This work was supported by the Canadian Institutes of Health Research, and the Princess Margaret Cancer Centre Foundation through grants held by C.J.P.
Authorship
Contribution: J.M.M., C.S.R., and C.J.P. conceived and designed the study; J.M.M., A.B., C.F., M.E.N., M.M., S.Y.C., and R.B. performed experiments; C.S.R. and R.B. conducted cecal punctures; and J.M.M. and C.J.P. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joshua M. Moreau, Princess Margaret Cancer Centre, 610 University Ave, Room 8-105, Toronto, ON, Canada, M5G 2M9; e-mail: josh.moreau@mail.utoronto.ca.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal