Key Points
Increasing receptor stability of an Mpl-based cell growth switch improves ex vivo expansion from cord blood CD34+ cells.
Expansion includes Epo-independent, macrophage-associated erythropoiesis from a novel erythroid-megakaryocytic precursor population.
Abstract
Several approaches for controlling hematopoietic stem and progenitor cell expansion, lineage commitment, and maturation have been investigated for improving clinical interventions. We report here that amino acid substitutions in a thrombopoietin receptor (Mpl)--containing cell growth switch (CGS) extending receptor stability improve the expansion capacity of human cord blood CD34+ cells in the absence of exogenous cytokines. Activation of this CGS with a chemical inducer of dimerization (CID) expands total cells 99-fold, erythrocytes 70-fold, megakaryocytes 0.5-fold, and CD34+ stem/progenitor cells 4.4-fold by 21 days of culture. Analysis of cells in these expanded populations identified a CID-dependent bipotent erythrocyte-megakaryocyte precursor (PEM) population, and a CID-independent macrophage population. The CD235a+/CD41a+ PEM population constitutes up to 13% of the expansion cultures, can differentiate into erythrocytes or megakaryocytes, exhibits very little expansion capacity, and exists at very low levels in unexpanded cord blood. The CD206+ macrophage population constitutes up to 15% of the expansion cultures, exhibits high-expansion capacity, and is physically associated with differentiating erythroblasts. Taken together, these studies describe a fundamental enhancement of the CGS expansion platform, identify a novel precursor population in the erythroid/megakaryocytic differentiation pathway of humans, and implicate an erythropoietin-independent, macrophage-associated pathway supporting terminal erythropoiesis in this expansion system.
Introduction
The ability to control the expansion, lineage commitment, and maturation of hematopoietic stem and progenitor cells (HSPCs), in a specific and regulated fashion, would provide a powerful tool for clinical intervention. The initial promise of recombinant cytokines for this purpose has been limited by their association with deleterious off-target effects.1-3 Currently, recombinant cytokines have proven useful for mobilizing HSPCs for collection by apheresis,4 treating anemia associated with chronic kidney disease and chemotherapy,5 and treating cancer-associated neutropenia.6 Cytokines support HSPC cell survival and proliferation in vitro during transduction with recombinant viral vectors,7 and support limited ex vivo expansion for improving outcomes in cord blood transplantation.8 Genetic engineering strategies based on drug resistance,9 or enhanced HSPC self-renewal,10 provide a means of controlling the expansion of specific cell populations, but require the use of cytotoxic drugs for selection or genes with oncogenic potential, raising both off-target and safety concerns.
We have been investigating an alternative approach for regulating hematopoietic cell expansion and differentiation based on the observation that signaling by many cytokine receptors is triggered by binding of 2 receptor molecules by a single cytokine molecule. By fusing the intracellular signaling domain of these receptors to an artificial dimerization domain, it is possible to bring receptor binding, and thus receptor signaling, under control of a small-drug molecule called a chemical inducer of dimerization (CID).3 Artificial cell growth switch (CGS) receptors of this type encoding the signaling domain of the thrombopoietin (TPO) receptor (Mpl) have proven especially useful for the regulated expansion of selected hematopoietic lineages in multiple settings.11-23 The 635-aa native Mpl protein, also known as CD110 and TPO-receptor, is a major regulator of megakaryocyte and platelet formation and has also been implicated in HSPC maintenance.24-26 Ex vivo culture and in vivo transplantation studies with constitutively active viral vectors expressing the artificially dimerizable version of this Mpl-based CGS receptor in HSPCs from mice,13-15 dogs,16,17 and humans18-23 demonstrated an unexpected and disproportionate effect of CID-mediated expansion on primitive erythroid cells, and to a lesser extent T and B lymphocytes, as well as megakaryocytes and platelets. In every instance, expansion was limited to cells transduced with the viral vector, and was reversible upon withdraw of the CID. Studies with high vector doses and highly purified HSPC populations provided evidence that this CGS system was capable of expanding HSPCs from human cord blood.21,22 However, most studies with cord blood CD34+ cells in culture, and all transplantation studies in mice and dogs, showed no evidence for CGS-mediated expansion of primitive HSPCs. Furthermore, efforts to use this system for cell expansion from adult sources of human HSPCs have also met with limited success.19
Although physiological levels of Tpo/Mpl signaling result in HSPC quiesence,25,26 superphysiological doses of Tpo induce HSPC replication in mice.26 Based on this observation, we hypothesized that the inability of this CGS system to expand primitive HSPC in most settings, and especially from adult human HSPCs, was the result of inadequate levels of CGS receptor signaling. To test this hypothesis, we substituted sequences in the Mpl signaling domain of the CGS receptor known to be involved in degradation of the human Mpl receptor, and assessed the expansion potential of the resulting constructs in human HSPC cultures. We describe here the capacity of one of these constructs to significantly improve the ex vivo expansion of both mature and immature hematopoietic populations from cord blood CD34+ cells. These studies also revealed a CD235a+/CD41a+ precursor population capable of differentiating into both erythrocytes and megakaryocytes similar to a population reported to arise during stress hematopoiesis in mice.27,28 This bipotent precursor population was found to undergo erythropoietin (Epo)–independent terminal erythropoiesis in contact with macrophages.
Methods
Lentiviral vectors
The self-inactivating CGS lentiviral vector LMFMPG was reported previously.23 The CGS receptor cassette is transcribed from the constitutively active murine stem cell virus (MSCV) promoter and is composed of a hybrid sequence encoding the modified binding domain FKBP(F36V) linked to complementary DNA (cDNA) encoding the intracellular domain of mouse Mpl. This hybrid protein also contains a myristoylation domain to target the inner cell membrane and a hemagglutinin (HA) tag. The green fluorescent protein (GFP) cassette is transcribed from the constitutively active PGK gene promoter. The hyperactive variants were created by introducing the following amino acid substitutions (coordinates based on the mouse Mpl sequence): K544F, K564F, and Y582F. Lentiviral vector lots were generated by cotransfection of 293T cells and concentrated 100-fold before use as described.29 Vector titers were determined by flow cytometric analysis of GFP expression in transduced HT1080 cells.
Ba/F3 transduction and expansion cultures
Murine Ba/F3 cells were vector-transduced and expanded in the absence of CID in RPMI 1640 media supplemented with 10% fetal bovine serum (RPMI/FBS) and 5% WEHI-conditioned medium as a source of interleukin-3 (IL-3) at 37°C, 5% CO2. After confirming similar rates of gene transfer using flow cytometry for GFP (wild type [wt], 40%; K*K* (K544F and K564F), 44%; Y* (Y582F), 39%; and K*K*Y*, 43%), cells were washed to remove IL-3 and then cultured in RPMI/FBS supplemented with the indicated concentrations of the CID drug AP1903, a derivative of AP20187 developed for clinical use (a gift from ARIAD Pharmaceuticals Inc). After 5 days, cell proliferation was assessed by the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay as previously described.11
Western blot analysis
Ba/F3 cell lines transduced with wt and Y* (Y582F) CGS vectors were expanded with 100 nM AP20187 until all cells expressed vector GFP. Equal numbers of cells were then plated in RPMI/FBS supplemented with cycloheximide at 0, 1, and 5 μg/mL. After 4 and 8 hours, cell lysates were prepared in the presence of protease and phosphatase inhibitors, resolved on 4% to 12% Bis-Tris gels, transferred to polyvinylidene difluoride membranes (NuPage system; Life Technologies), and probed with a primary antibody to the CGS receptor HA tag followed by an anti-mouse horseradish peroxidase–conjugated secondary antibody. Blots were developed and exposed using enhanced chemiluminescence and Hyperfilm (GE Healthcare). Band densities were quantified using Image J software (http://rsb.info.nih.gov/ij/). Analysis was only performed on this CGS variant because it provided the best expansion in primary CD34+ cell cultures.
Primary CD34+ purification, transduction, and expansion cultures
Anonymized human cord blood was obtained from the Puget Sound Blood Center Cord Blood Program following donor consent. Mononuclear cells were enriched for CD34+ cells by immunomagnetic separation (Miltenyi Biotec Inc) to an average 84% ± 5% purity. Fresh CD34+ cells were prestimulated in Iscove modified Dulbecco medium supplemented with 10% FBS (IMDM/FBS), 50 ng/mL recombinant human (rh) stem cell factor (rhSCF), 20 ng/mL rhIL-6, 20 ng/mL rhTPO, and 100 ng/mL rhFlt3-L at 37°C, 5% CO2. All cytokines were purchased from PeproTech. After 3 hours, the cultures were further supplemented with 5 μg/mL polybrene and CGS vectors at a multiplicity of infection of 10, and incubated overnight. The cells were then washed and replated in IMDM/FBS and 100 nM AP20187 without additional cytokines at a dose of ∼5 × 105 cells per mL per well. At days 3, 7, 11, 14, 21, and 26 cells were collected, washed, and either saved for analysis or replated in fresh medium at a target concentration of 5 × 105 cells per mL. Anonymized granulocyte–colony-stimulating factor (G-CSF) mobilized peripheral blood CD34+ cells were obtained from the Fred Hutchinson Cancer Research Center Hematopoietic Cell Processing Core following donor consent, and were transduced and cultured in the same fashion as the cord blood CD34+ cells, except that they were prestimulated for 24 hours to allow for recovery from cryopreservation.
Cell analysis
Cells were stained with fluorochrome-conjugated antibodies (CD34–phycoerythrin [PE] Cy7a, CD38-PE, CD41a-PE, CD42b–allophycocyanin [APC], CD45RA-APC, CD90-APC, CD123-PECy7a, and CD235a-APC; BD Biosciences), and analyzed for antibody staining and vector GFP expression on a FACSCanto 6-color flow cytometer (BD Biosciences) using FloJo software. Data were collected from a target cell count ≥30 000, dead cells were excluded by 7-aminoactinomycin D (7-AAD) staining, nucleated cells were gated based on light scatter, and gates were determined by untransduced, unstained, or isotype controls. Sorting was performed on a FACSAria cell sorter (BD Biosciences). Cytospins were prepared by suspending cells in 200 µL of phosphate-buffered saline (PBS)/2% FBS, and centrifuging onto slides at 72 g for 5 minutes. Air-dried slides were Wright-Giemsa stained at the Core Center of Excellence in Hematology cell characterization resource at the Fred Hutchinson Cancer Research Center and imaged at ×200 magnification.
Secondary cultures
Colony-forming progenitor assays were performed using MethoCult H4034 Optimum (StemCell Technologies). Plates were incubated at 37°C, 5% CO2, and scored for colony formation and classification for up to 18 days. To assess erythroid differentiation potential, sorted cells were cultured in IMDM/FBS supplemented with 2 IU/mL rhEpo ± 50 ng/mL rhSCF. To assess megakaryocyte differentiation potential, sorted cells were cultured in IMDM supplemented with 20% bovine serum albumin, insulin and transferrin (BIT) serum substitute (StemCell Technologies), 30 ng/mL rhTPO, 50 ng/mL rhIL-6, 13.5 ng/mL rhIL-9, ± 1 ng/mL rhSCF as described.30
Results
Derivation and validation of hyperactive variants of the Mpl-based CGS
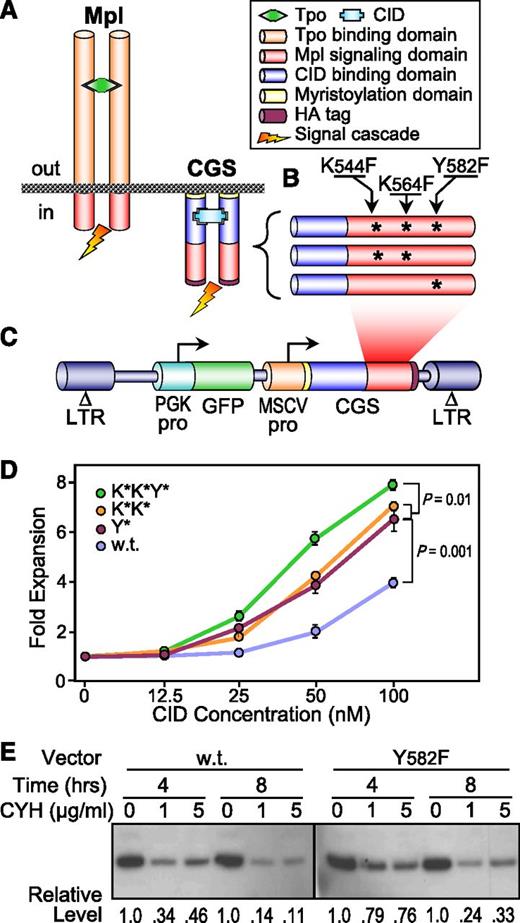
Toward the goal of improving the signaling activity of our Mpl-based CGS (Figure 1A), we introduced single amino acid substitutions at 3 specific sites in the Mpl intracellular domain shown by others to be involved in degradation of the homologous human Mpl receptor (Figure 1B). This included 2 sites involved in protein ubiquitination (K544F and K564F, K*K*),31 and 1 site bound by the accessory protein AP2 leading to receptor endocytosis (Y582F, Y*).32 The resulting variants were introduced into a lentiviral vector coexpressing a GFP reporter gene (Figure 1C),22 and assessed for CGS activity in factor-dependent Ba/F3 cells. As seen in Figure 1D, all 3 variants reduced the threshold for CID responsiveness (from 50 nM to 25 nM) and increased the proliferative magnitude of CID responsiveness (from threefold to nearly sixfold at 100 nM CID) compared with the vector containing the wt Mpl signaling domain. Western blotting confirmed that the AP2 binding site–deficient variant increased the half-life of the CGS receptor protein by 34%, from 4.3 to 5.8 hours (Figure 1E).
Derivation and validation of hyperactive CGS variants. (A) Schematic comparing the principal components of the Tpo receptor Mpl and the hybrid Mpl-based CGS receptor. Tpo and CID not to scale. (B) Schematic indicating the positions of the amino acid substitutions introduced into the Mpl signaling domain of the CGS receptor. (C) Design of the lentiviral vectors expressing the CGS variants Y* (Y582F), K*K* (K544F and K564F), and K*K*Y*. Coordinates are based on amino acid sequence of mouse Mpl. (D) Hyperactive variants improve CGS-mediated expansion of Ba/F3 cells. Murine Ba/F3 cells were transduced with lentivectors expressing the CGS containing the wt, Y*, K*K*, or K*K*Y* Mpl signaling domains. After confirming similar rates of gene transfer, cells were cultured in the indicated concentration of the CID AP1903. After 5 days, cell proliferation was assessed by the MTS assay. Data represent the mean ± SD of triplicate determinations. P values are based on the t test across all concentrations. (E) The hyperactive variant Y* improves CGS receptor stability. Ba/F3 cells transduced with the wt or Y* CID lentivectors and expanded in 100 nM CID AP20187 were treated with CYH for 4 and 8 hours, and the level of wt and Y* CID receptor proteins was compared with untreated controls (0 μg/mL CYH) by western blotting using an HA tag antibody. The relative levels of CGS protein compared with the untreated controls are shown at the bottom. ΔLTR, self-inactivating long-terminal repeat; CYH, cycloheximide; GFP, GFP reporter gene; MSCVpro, MSCV promoter; PGKpro, PGK gene promoter; SD, standard deviation.
Derivation and validation of hyperactive CGS variants. (A) Schematic comparing the principal components of the Tpo receptor Mpl and the hybrid Mpl-based CGS receptor. Tpo and CID not to scale. (B) Schematic indicating the positions of the amino acid substitutions introduced into the Mpl signaling domain of the CGS receptor. (C) Design of the lentiviral vectors expressing the CGS variants Y* (Y582F), K*K* (K544F and K564F), and K*K*Y*. Coordinates are based on amino acid sequence of mouse Mpl. (D) Hyperactive variants improve CGS-mediated expansion of Ba/F3 cells. Murine Ba/F3 cells were transduced with lentivectors expressing the CGS containing the wt, Y*, K*K*, or K*K*Y* Mpl signaling domains. After confirming similar rates of gene transfer, cells were cultured in the indicated concentration of the CID AP1903. After 5 days, cell proliferation was assessed by the MTS assay. Data represent the mean ± SD of triplicate determinations. P values are based on the t test across all concentrations. (E) The hyperactive variant Y* improves CGS receptor stability. Ba/F3 cells transduced with the wt or Y* CID lentivectors and expanded in 100 nM CID AP20187 were treated with CYH for 4 and 8 hours, and the level of wt and Y* CID receptor proteins was compared with untreated controls (0 μg/mL CYH) by western blotting using an HA tag antibody. The relative levels of CGS protein compared with the untreated controls are shown at the bottom. ΔLTR, self-inactivating long-terminal repeat; CYH, cycloheximide; GFP, GFP reporter gene; MSCVpro, MSCV promoter; PGKpro, PGK gene promoter; SD, standard deviation.
Hyperactive CGS variants improve expansion from human CD34+ cells
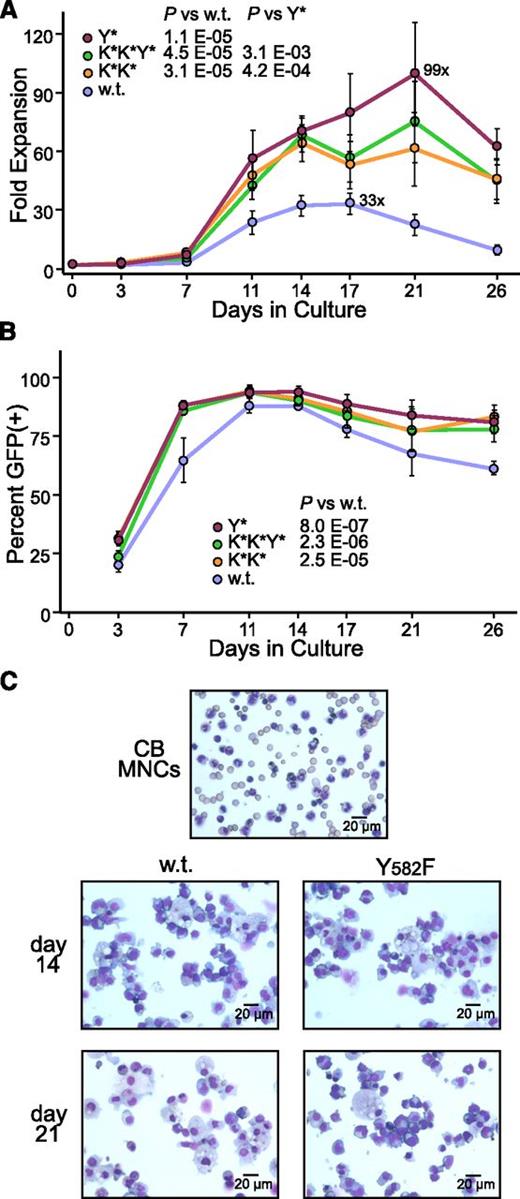
Culture studies with human cord blood CD34+ cells also demonstrated a pronounced improvement in the proliferative magnitude of CID responsiveness for all 3 of the hyperactive CGS variants (Figure 2A). In particular, the Y* CGS variant increased the total cell output, from 33-fold to 99-fold. Based on an average initial transduction rate of ∼25%, this translates to a degree of expansion for cells transduced with the Y* CGS vector of nearly 400-fold. Tracking of the percentage of cells expressing vector GFP further confirmed that this increase in total cell output was due to the accelerated expansion of vector-transduced cells, peaking at roughly 90% of all cells (Figure 2B). Cytospins confirmed the expansion products generated by both the wt and Y* CGS vectors were dominated by cells of the erythroid lineage characterized by dark blue cytoplasm, dark nuclei, and prominent Golgi, representing different stages of maturation, as well as macrophages characterized by blue nuclei, pale foamy cytoplasm, and a small nucleus to cytoplasm ratio (Figure 2C). Titration studies demonstrated that increasing the dose of the wt CGS vector increased the amount of expansion, but this effect plateaued at a level that was still statistically less than the amount of expansion achieved with a standard dose of the Y* CGS variant (supplemental Figure 1, see supplemental Data available at the Blood Web site). This in turn suggests that the Y* variant improves expansion not only through increased receptor stability, but also through a functional improvement in receptor signaling. The improved activity of the Y* CGS vector also allowed for a greater than fourfold improvement in the expansion from adult hematopoietic CD34+ cells compared with the wt CGS vector (supplemental Figure 2A-B), a cell population which is relatively resistant to CGS-mediated expansion.
Hyperactive variants improve CGS-mediated expansion from cord blood CD34+ cells. (A) Expansion of total cells. Cord blood-derived CD34+ cells were transduced with lentivectors expressing the wt, Y*, K*K*, and K*K*Y* variants of the CGS and expanded in serum-containing medium supplemented with 100 nM CID AP20187 and no additional cytokines. Cell numbers were determined by hemocytometer count with media changes on the indicated days, and are reported as a fold-expansion based on the starting cell number. The maximum fold-expansion is shown for the wt and Y* samples. P values are based on the t test across all time points. P = .16 for K*K* vs K*K*Y*. (B) Expansion of transduced cells. The percent of cells expressing vector GFP was also determined on the indicated days by flow cytometry as a means of determining the degree of expansion for the vector-transduced cells. Data represent the mean ± standard error from 4 independent experiments. P values are based on the t test across all time points. (C) Morphology of expanded cells. Representative Wright-Giemsa–stained cytospins are shown for the original cord blood mononuclear cells (CB MNC) used to prepare the CD34+ cells, and from day 14 and day 21 cultures expanded with the wt and Y* CGS vectors.
Hyperactive variants improve CGS-mediated expansion from cord blood CD34+ cells. (A) Expansion of total cells. Cord blood-derived CD34+ cells were transduced with lentivectors expressing the wt, Y*, K*K*, and K*K*Y* variants of the CGS and expanded in serum-containing medium supplemented with 100 nM CID AP20187 and no additional cytokines. Cell numbers were determined by hemocytometer count with media changes on the indicated days, and are reported as a fold-expansion based on the starting cell number. The maximum fold-expansion is shown for the wt and Y* samples. P values are based on the t test across all time points. P = .16 for K*K* vs K*K*Y*. (B) Expansion of transduced cells. The percent of cells expressing vector GFP was also determined on the indicated days by flow cytometry as a means of determining the degree of expansion for the vector-transduced cells. Data represent the mean ± standard error from 4 independent experiments. P values are based on the t test across all time points. (C) Morphology of expanded cells. Representative Wright-Giemsa–stained cytospins are shown for the original cord blood mononuclear cells (CB MNC) used to prepare the CD34+ cells, and from day 14 and day 21 cultures expanded with the wt and Y* CGS vectors.
Characterization of CGS expansion products
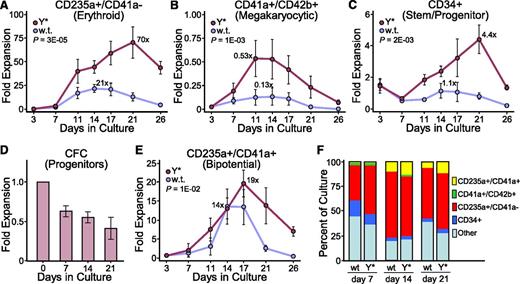
Based on the starting cell number, CD235a+/CD41a− erythroid cells were expanded 70-fold by the Y*CGS vector compared with only 21-fold by the wt CGS vector (Figure 3A). Although the output of CD41a+/CD42b+ megakaryocytes was only 0.5-fold when compared with the total number of starting cells, a comparison of this specific subset of cells at day 3 vs day 11 of culture revealed a 22-fold expansion for the Y* CGS vector, compared with fivefold for the wt CGS vector (Figure 3B). The wt CGS vector provided for a stable level of CD34+ cells in these cultures, whereas the Y* CGS vector expanded the number of CD34+ cells 4.4-fold by 21 days of culture (Figure 3C). This was correlated with a maintenance of about half of the initial progenitor colony-forming potential for the Y* CGS vector (Figure 3D), whereas the number of colony-forming cells declined to undetectable levels with the wt CGS vector (data not shown). During the course of this analysis, we noted the expansion of a novel cell population in these cultures staining for both CD235a and CD41a. Expression of CD235a (glycophorin A) is strictly associated with erythrocytes, erythroblasts, and their immediate precursors,33,34 whereas CD41a (integrin αIIb) is associated with megakaryocytes and platelets,35 as well as some other more primitive cells.36,37 Both the absolute level of expansion (19-fold) and the period of positive expansion (17 days) for this CD235a+/CD41a+ population was greater with the Y* CGS vector compared with the wt CGS vector (Figure 3E). The Y* CGS vector also expanded CD235a+/CD41a+ cells from adult hematopoietic CD34+ cells to a greater degree than the wt CGS vector (supplemental Figure 2C). As summarized in Figure 3F, the increased expansion from cord blood CD34+ cells provided by the Y* CGS vector was distributed across all of the cell types analyzed, with a moderate preference for CD235a+ erythroid cells at day 7, CD41a+/CD42b+ megakaryocytes at day 14, and CD235a+/CD41a+ cells at day 21 compared with the wt CGS vector. Additional studies demonstrated that all 3 of these populations were predominantly positive for vector GFP, indicating their expansion was CGS-dependent (supplemental Figure 3A). Furthermore, quantitative polymerase chain reaction (PCR) analysis indicated that the residual GFP-negative cells present in later-stage cultures did not contain vector provirus, indicating that expansion of the CD235a−/CD41a− population was CGS-independent (supplemental Figure 3B).
Hyperactive CGS variant improves expansion of both mature and primitive cells. Expansion cultures were established with cord blood CD34+ cells transduced with lentivectors expressing the wt or Y* CGS as for Figure 2. Cell aliquots were analyzed by immunofluorescent staining and flow cytometry for (A) erythroid cells (CD235a+/CD41a−), (B) megakaryocytic cells (CD41a+/CD42b+), (C) primitive stem/progenitor cells (CD34+), and (E) bipotent erythroid/megakaryocytic precursor cells (CD235a+/CD41a+), later determined to be PEMs. Data represent the mean ± standard error (SE) from 4 independent experiments for the fold-expansion based on the starting number of total cells. P values are based on the t test across all time points. The maximum fold-expansion is shown for the wt and Y* samples based on each parameter. (D) Histograms showing the fold expansion of progenitor colony-forming cells present in the cord blood CD34+ expansion cultures transduced with the Y* lentivector and expanded for the indicated days. Colonies were detected by plating cell aliquots in methylcellulose supplemented with a broad cytokine cocktail, and consisted predominantly of primitive and mature erythroid colonies, granulocyte-macrophage colonies, and low levels of megakaryocyte colonies and colonies with mixed phenotypes. Data represents the mean ± SE from 3 independent experiments. (F) Histograms showing the relative frequency of the indicated cell populations present in the cord blood CD34+ cultures transduced with the wt or Y* lentivectors and expanded for 7, 14, and 21 days.
Hyperactive CGS variant improves expansion of both mature and primitive cells. Expansion cultures were established with cord blood CD34+ cells transduced with lentivectors expressing the wt or Y* CGS as for Figure 2. Cell aliquots were analyzed by immunofluorescent staining and flow cytometry for (A) erythroid cells (CD235a+/CD41a−), (B) megakaryocytic cells (CD41a+/CD42b+), (C) primitive stem/progenitor cells (CD34+), and (E) bipotent erythroid/megakaryocytic precursor cells (CD235a+/CD41a+), later determined to be PEMs. Data represent the mean ± standard error (SE) from 4 independent experiments for the fold-expansion based on the starting number of total cells. P values are based on the t test across all time points. The maximum fold-expansion is shown for the wt and Y* samples based on each parameter. (D) Histograms showing the fold expansion of progenitor colony-forming cells present in the cord blood CD34+ expansion cultures transduced with the Y* lentivector and expanded for the indicated days. Colonies were detected by plating cell aliquots in methylcellulose supplemented with a broad cytokine cocktail, and consisted predominantly of primitive and mature erythroid colonies, granulocyte-macrophage colonies, and low levels of megakaryocyte colonies and colonies with mixed phenotypes. Data represents the mean ± SE from 3 independent experiments. (F) Histograms showing the relative frequency of the indicated cell populations present in the cord blood CD34+ cultures transduced with the wt or Y* lentivectors and expanded for 7, 14, and 21 days.
Characterization of the bipotent CD235a+/CD41a+ PEM population
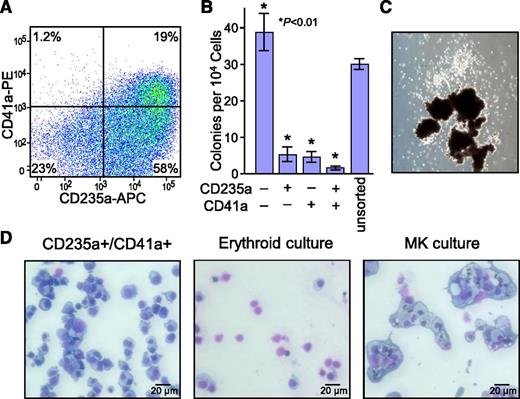
To assess the expansion and differentiation potential of the novel CD235a+/CD41a+ population, cells from day 14 to 21 cord blood CD34+ cultures expanded with the Y* CGS vector were sorted based on their expression pattern (Figure 4A). Subsequent clonogenic assays in methylcellulose demonstrated that almost all of the colony-forming progenitor cells were located in the CD235a−/CD41a− population (Figure 4B), and that the few colonies that grew from the CD235a+/CD41a+ population frequently formed primitive CFU-Mix colonies containing both CD235a+ erythroid- and CD41a+/CD42b+ megakaryocyte-derived cells (Figure 4C). This is consistent with the observation that almost all of the CD34+ cells were contained within the CD235a−/CD41a− population. Secondary cultures in cytokine cocktails demonstrated that CD235a+/CD41a+ cells had the capacity to differentiate into both erythrocytes and megakaryocytes (Figure 4D). This differentiation was not associated with expansion of cell numbers, occurred maximally within 2 days, and was not dependent on SCF (supplemental Figure 4). These are the same properties ascribed previously by others to a Ter119+/CD42d+ bipotent population arising during stress hematopoiesis in mice and named a precursor for erythrocytes and megakaryocytes (PEM).27,28 Subsequent studies demonstrated that the CD235a+/CD41a+ cells in the CGS expansion cultures were predominantly negative for CD34 and CD123 (Figure 5A), both markers associated with primitive progenitors. Analysis of mononuclear cells from 6 cord blood samples indicated the presence of a similar CD235a+/CD41a+ population at a frequency of 0.27% ± 0.15% (range, 0.11%-0.49%; see Figure 5B for example), or about half the frequency of PEM in bone marrow of untreated mice.28 PEM cells from the CGS expansion cultures and from fresh cord blood were also predominantly negative for CD38, CD90, and the SCF receptor CD117, 3 other markers associated with primitive HSPCs (supplemental Table 1). Real-time PCR analysis demonstrated that PEM sorted from Y* CGS-expanded cord blood expressed genes associated with both the erythroid and megakaryocytic lineages, including high levels of the erythropoietin receptor, low to moderate levels of α-globin, high levels of CD41, but almost no detectable receptor for the megakaryocyte-specific genes MPL or PF4, consistent with the properties of PEM from mice (supplemental Figure 5).27 This same analysis also demonstrated that PEM from expansion cultures expressed an even higher ratio of fetal γ-globin than primary erythroid cells from cord blood (supplemental Figure 6). However, the overall levels of all globin transcripts in the PEM were lower than the levels found in CD235a+/CD41a− cells collected from cord blood, and higher than the levels found in CD235a+/CD41a− cells collected from the CGS expansion cultures.
Differentiation potential of bipotent CD235a+/CD41a+ PEM. (A) Representative flow cytometric histogram demonstrating the CD235a+/CD41a+ immunofluorescent staining pattern of cord blood CD34+ cells transduced with the Y* CGS lentiviral vector and expanded for 14 days in 100 nM AP20187. Gates were set based on staining controls. The percent of cells in each quadrant are indicated. (B) Relative progenitor content of CD235a/CD41a subpopulations. Cells were sorted from day 14 to 21 cord blood CD34+ cultures expanded with the Y* CGS lentiviral vector based on presence or absence of CD235a and CD41a expression, and plated in methylcellulose cultures supplemented with a broad cytokine cocktail. Data represent the mean ± SD from 3 independent experiments. P values are based on the t test vs the unsorted control. (C) Example of a mixed progenitor colony generated from sorted CD235a+/CD41a+ PEM, showing the presence of both erythroid bursts (dark globules at bottom) and nonerythroid cells (white colony at top). (D) Differentiation potential of PEM. CD235a+/CD41a+ PEM were sorted from day 14 cord blood CD34+ Y* CGS expansion cultures and transferred to media supporting either erythroid (Epo + SCF) or megakaryocytic (TPO, IL-6, IL-9, SCF) differentiation. After 6 days, the cells were collected, prepared as cytospins, and stained by Wright-Giemsa. Left, sorted cells before secondary culture characterized morphologically as erythroid precursors; middle, representative of erythroid cultures characterized morphologically as enucleated red blood cells and a few pre-erythrocytes; and right, representative of megakaryocyte cultures characterized morphologically as megakaryocytes exhibiting mono- and polylobular nuclei and surrounded by a halo of platelets, and confirmed as CD41a+/CD42b+ by immunofluorescent staining and flow cytometry.
Differentiation potential of bipotent CD235a+/CD41a+ PEM. (A) Representative flow cytometric histogram demonstrating the CD235a+/CD41a+ immunofluorescent staining pattern of cord blood CD34+ cells transduced with the Y* CGS lentiviral vector and expanded for 14 days in 100 nM AP20187. Gates were set based on staining controls. The percent of cells in each quadrant are indicated. (B) Relative progenitor content of CD235a/CD41a subpopulations. Cells were sorted from day 14 to 21 cord blood CD34+ cultures expanded with the Y* CGS lentiviral vector based on presence or absence of CD235a and CD41a expression, and plated in methylcellulose cultures supplemented with a broad cytokine cocktail. Data represent the mean ± SD from 3 independent experiments. P values are based on the t test vs the unsorted control. (C) Example of a mixed progenitor colony generated from sorted CD235a+/CD41a+ PEM, showing the presence of both erythroid bursts (dark globules at bottom) and nonerythroid cells (white colony at top). (D) Differentiation potential of PEM. CD235a+/CD41a+ PEM were sorted from day 14 cord blood CD34+ Y* CGS expansion cultures and transferred to media supporting either erythroid (Epo + SCF) or megakaryocytic (TPO, IL-6, IL-9, SCF) differentiation. After 6 days, the cells were collected, prepared as cytospins, and stained by Wright-Giemsa. Left, sorted cells before secondary culture characterized morphologically as erythroid precursors; middle, representative of erythroid cultures characterized morphologically as enucleated red blood cells and a few pre-erythrocytes; and right, representative of megakaryocyte cultures characterized morphologically as megakaryocytes exhibiting mono- and polylobular nuclei and surrounded by a halo of platelets, and confirmed as CD41a+/CD42b+ by immunofluorescent staining and flow cytometry.
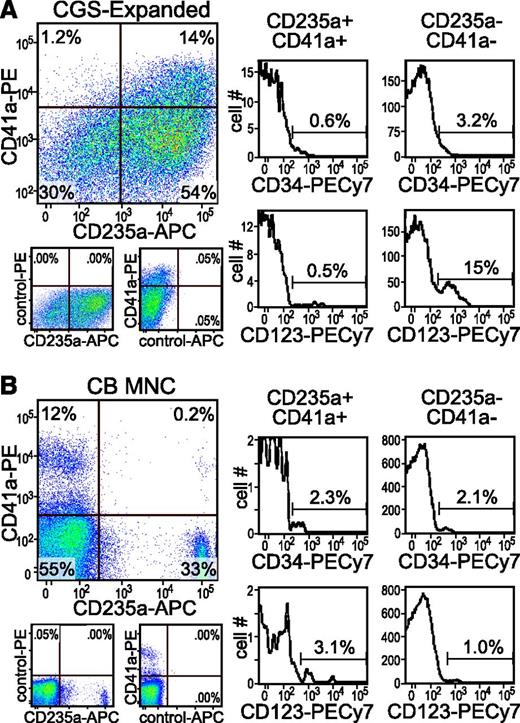
Identification of PEM population in unexpanded cord blood. Cells collected from day 14 cord blood CD34+ Y* CGS expansion cultures (A) and fresh CB MNCs (B) were analyzed by immunofluorescent staining and flow cytometry for CD235a+/CD41a+ PEM and coexpression of the primitive hematopoietic markers CD34 and CD123. Gates were set based on isotype staining controls (see examples shown under the 2-color plots). The percent of cells in each quadrant or gate are indicated.
Identification of PEM population in unexpanded cord blood. Cells collected from day 14 cord blood CD34+ Y* CGS expansion cultures (A) and fresh CB MNCs (B) were analyzed by immunofluorescent staining and flow cytometry for CD235a+/CD41a+ PEM and coexpression of the primitive hematopoietic markers CD34 and CD123. Gates were set based on isotype staining controls (see examples shown under the 2-color plots). The percent of cells in each quadrant or gate are indicated.
Characterization of macrophages in cord blood CD34+ expansion cultures
Macrophages play a central role in normal erythrocyte maturation, forming the basis of erythroid islands found in normal bone marrow.38 Cytospin analysis revealed the presence of macrophages in CGS expansion cultures, and that these macrophages were often physically surrounded by maturing erythroblasts (Figure 6A). Immunofluorescent staining and flow cytometry (Figure 6B), as well as sorting and cytospin studies (Figure 6C), confirmed the presence of activated CD206+ macrophages in late-stage cultures expanded with the Y* CGS. Subsequent tracking studies demonstrated that the relative frequency of these CD206+ macrophages increases sharply around day 7 of culture, coincident with the onset of active erythropoiesis, but then declines to a maintenance level of 2% to 3% of all cells in the cultures (Figure 6D). Surprisingly, the relative frequency of CD206+ macrophages expressing vector GFP remains unchanged throughout the culture (Figure 6B,D), whereas the total number of CD206+ cells expands about 110-fold by day 26 of culture (data not shown). As such, it appears that CD206+ macrophages, or a macrophage progenitor/precursor populations, undergo a substantial degree of CGS-independent expansion in these cultures.
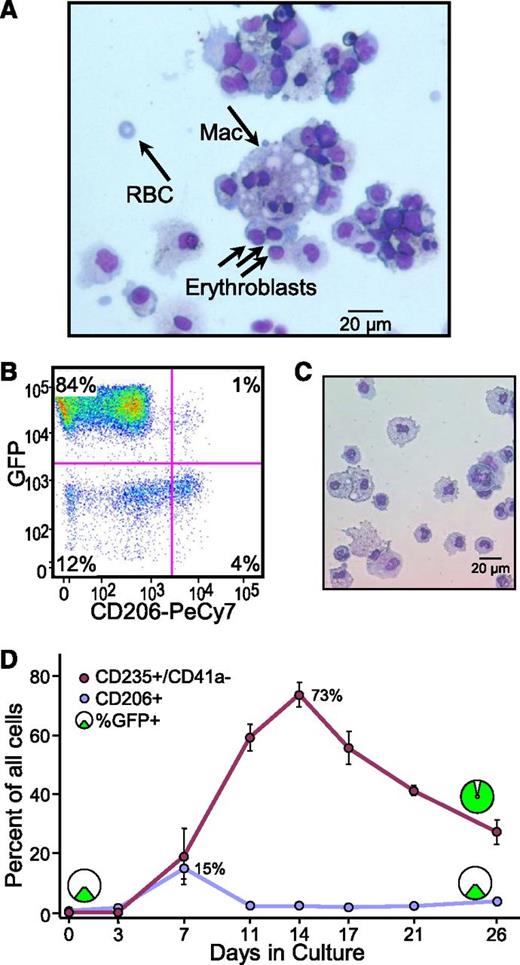
Macrophages are passengers in CGS expansion cultures. (A) Cytopathology evidence for macrophages. Cytospins were prepared from day 14 cord blood CD34+ Y* CGS expansion cultures and stained by Wright-Giemsa. The representative slide shows the presence of morphologically distinct macrophage (Mac) and their tight physical association with developing erythroblasts reminiscent of erythroid islands. The presence of an enucleated RBC is also indicated. (B) Immunophenotyping evidence for macrophages. Cells from day 14 cord blood CD34+ Y* CGS expansion cultures were stained for the activated macrophage cell surface marker CD206 and analyzed by flow cytometry. The representative histogram shows the presence of 5% CD206+ cells, and that only 20% of these also express viral vector GFP. Gates were set based on staining controls. The percentage of cells in each quadrant is indicated. (C) Cytopathology of CD206+ cells. Cells expressing CD206 were sorted and analyzed by cytospin and Wright-Giemsa staining. The representative slide shows a preponderance of large macrophages with large foamy cytoplasm and blue nuclei. (D) Contribution of macrophages to expansion cultures. The relative frequency of CD235a+/CD41a− erythroid cells and activated CD206+ macrophages were tracked over time in cord blood CD34+ cultures expanded with the Y* CGS by immunofluorescent staining and flow cytometry. The relative frequency of cells expressing vector GFP is shown for the 2 populations early and late in culture. Data represent the mean ± SE from 3 independent experiments. P values are based on the t test across all time points.
Macrophages are passengers in CGS expansion cultures. (A) Cytopathology evidence for macrophages. Cytospins were prepared from day 14 cord blood CD34+ Y* CGS expansion cultures and stained by Wright-Giemsa. The representative slide shows the presence of morphologically distinct macrophage (Mac) and their tight physical association with developing erythroblasts reminiscent of erythroid islands. The presence of an enucleated RBC is also indicated. (B) Immunophenotyping evidence for macrophages. Cells from day 14 cord blood CD34+ Y* CGS expansion cultures were stained for the activated macrophage cell surface marker CD206 and analyzed by flow cytometry. The representative histogram shows the presence of 5% CD206+ cells, and that only 20% of these also express viral vector GFP. Gates were set based on staining controls. The percentage of cells in each quadrant is indicated. (C) Cytopathology of CD206+ cells. Cells expressing CD206 were sorted and analyzed by cytospin and Wright-Giemsa staining. The representative slide shows a preponderance of large macrophages with large foamy cytoplasm and blue nuclei. (D) Contribution of macrophages to expansion cultures. The relative frequency of CD235a+/CD41a− erythroid cells and activated CD206+ macrophages were tracked over time in cord blood CD34+ cultures expanded with the Y* CGS by immunofluorescent staining and flow cytometry. The relative frequency of cells expressing vector GFP is shown for the 2 populations early and late in culture. Data represent the mean ± SE from 3 independent experiments. P values are based on the t test across all time points.
Discussion
Incorporating the Mpl signaling domain into the CGS platform allows for the expansion of a much wider range of hematopoietic lineages than can be achieved with native Tpo/Mpl. There are likely 2 main reasons for this difference: (1) native Mpl is predominantly expressed on primitive HSPCs and megakaryocytes, whereas the CGS vector is expressed constitutively; and (2) native Mpl signaling is downregulated in part by internalization of dimerized receptors, whereas CGS receptor signaling occurs intracellularly. In addition, native Mpl signaling is difficult to block in vivo due to circulating Tpo, whereas CGS signaling can be tightly regulated by the addition or withdrawal of the CID while avoiding off-target effects. Also, unlike native Mpl, expansion by this CGS platform has an inherent bias for the erythroid lineage, followed by megakaryocytes/platelets, a subset of myeloid cells, and when assessed in vivo, lymphoid cells.13-18,22,23 These properties constitute fundamental differences between native Mpl (including studies with viral vectors expressing native Mpl),39 and our Mpl-based CGS platform. The studies presented here suggest that the Mpl-based CGS receptor is subject to the same receptor endocytosis pathways governing native Mpl. However, it appears that the link between blocking receptor degradation and an increased capacity for cellular proliferation may be more complicated than originally envisioned31,32 because the improved expansion achieved by the hyperactive variants cannot be fully recapitulated by simply increasing the dose of the wt CGS receptor. Future studies will be needed to identify the mechanism(s) underlying this increase in receptor signaling, to determine why the Y* variant performed statistically better than the K*K*Y* variant, and to determine whether similar changes occur in the setting of native Mpl.
We found that these modifications to the Mpl signaling domain of the CGS receptor substantially improved the overall activity of this CGS receptor across all hematopoietic lineages, including CD34+ cells and colony-forming progenitors. These effects are consistent with the observation that treating mice with low doses of exogenous Tpo induced HSPC quiescence, whereas similar studies with superphysiological doses of Tpo resulted in HSPC expansion.25,26 Although the erythroid and megakaryocytic cells generated in the expansion cultures described here were not directly assessed for functionality in vivo, previous studies in mice and dogs demonstrated that erythrocytes and platelets generated by the CGS in vivo exhibit the same half-lives as their normal counterparts and do not exhibit gross physiological defects.13-18,22,23
The PEM population identified in cord blood and adult CD34+ cultures expanded with the Y* CGS receptor shares several properties with the PEM population described by others in association with stress hematopoiesis in mice.27,28 Both populations simultaneously express cell surface markers typically associated with terminal erythropoiesis and megakaryopoiesis, but are negative for markers typically associated with HSPCs (CD34/ScaI). Both populations exhibit the bipotent capacity to differentiate into either erythrocytes or megakaryocytes, very little progenitor colony-forming ability, and gene expression patterns that represent a mix of erythroid and megakaryocytic profiles but with a clear erythroid bias. Finally, both populations are present, albeit at low levels (<1%) under steady-state conditions in vivo. These similarities suggest that the PEM arising in CGS-expanded cultures, as well as the erythrocytes dominating these cultures, arise through the mechanism(s) underlying stress hematopoiesis. This is consistent with the retention of a high ratio of fetal to adult hemoglobin in both the PEM and their progeny.
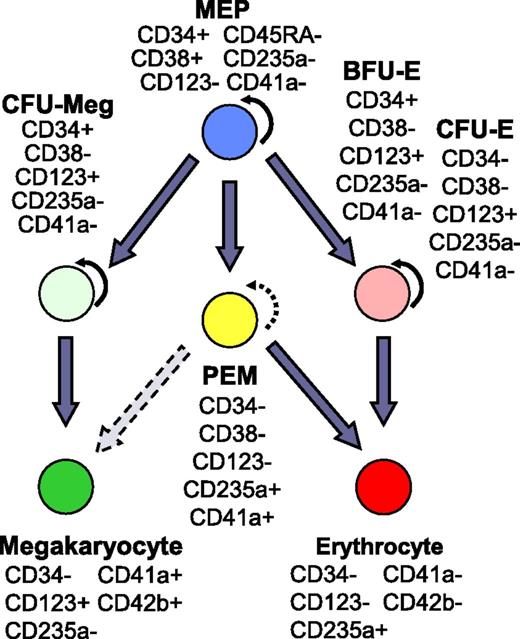
Based on the similarities to mouse PEM, and the characterization presented here, we propose that PEM represent an ontologically distinct population in the erythroid/megakaryocytic arm of human hematopoiesis. As summarized in Figure 740-45 , PEM do not express the primitive cell surface marker CD34 characteristic of megakaryocyte-erythrocyte progenitors (MEPs), colony-forming unit-megakaryocytes (CFU-Meg), or burst-forming unit-erythroid (BFU-E). However, PEM do share the CD123− phenotype characteristic of MEP, but not CFU-Meg, BFU-E, or CFU-erythroid (CFU-E). Based on these properties, the lack of colony-forming ability, and the expression of markers and genes associated with terminal erythropoiesis and megakaryopoiesis, we propose that PEM bypass the CFU-Meg and BFU-E/CFU-E classical pathways, and instead arise directly from MEP as a shortcut to generate red blood cells (RBCs) and megakaryocytes under conditions of stress. The observation that PEM also constitute a major component of the cultures expanded by the Y* CGS from adult-derived CD34+ cells indicates that PEM are not restricted to cord blood, but instead likely represent a previously unidentified population in definitive human hematopoiesis.
PEM population in the hierarchy of human hematopoiesis. The schema indicates the proposed placement of PEM in the erythrocyte-megakaryocyte differentiation arm of human hematopoiesis. The expression patterns of key CD antigens are shown for principal cell populations. Curved arrow, self-renewal capacity; straight arrow, differentiation path; broken arrows, minimal self-renewal or differentiation potential. References for progenitor-specific markers: MEP33-35,40,41 ; CFU-Meg30,33-35,41,42 ; CFU-E/BFU-E.33-35,41,43-45 CD, cluster of differentiation.
PEM population in the hierarchy of human hematopoiesis. The schema indicates the proposed placement of PEM in the erythrocyte-megakaryocyte differentiation arm of human hematopoiesis. The expression patterns of key CD antigens are shown for principal cell populations. Curved arrow, self-renewal capacity; straight arrow, differentiation path; broken arrows, minimal self-renewal or differentiation potential. References for progenitor-specific markers: MEP33-35,40,41 ; CFU-Meg30,33-35,41,42 ; CFU-E/BFU-E.33-35,41,43-45 CD, cluster of differentiation.
The importance of macrophages in these cultures remains unclear. They surge in number during the onset of large-scale erythropoiesis in the expansion cultures, interact directly with maturing erythrocytes, and appear to form erythroid islands reminiscent of those found in bone marrow. Furthermore, these macrophages, or progenitors giving rise to these macrophages, are expanding in a CGS-independent manner in the CID-treated cultures, but not in CID-untreated cultures. This in turn suggests that the macrophages are expanding in response to the CGS-driven production of CFU-E and/or their physical interactions with maturing erythrocytes arising from these CFU-E. As such, this CGS-based culture system may prove valuable for dissecting both the mechanisms supporting the macrophages at the heart of erythroid islands, and the mechanisms capable of supporting Epo-independent terminal erythropoiesis in this setting.
Optimizing the activity of the Mpl-based CGS receptor and understanding the cellular components of the associated expansion cultures are critical for translating the CGS expansion platform to the clinic. The studies reported here describe a fundamental enhancement of the CGS expansion platform, identify a novel precursor population in the erythroid/megakaryocyte differentiation pathway of humans that constitutes a major component of the CGS expansion cultures, and point to a potentially important role for macrophages in these expansion cultures. Although some CGS-based cultures have been reported to support the expansion of primitive hematopoietic cells,15,19,21,22 the cultures described here do not support the large-scale expansion of primitive HSPCs necessary for enhancing long-term engraftment. However, the large-scale expansion of cells committed to the erythroid/megakaryocytic lineage could be considered as a means of transiently supporting patients undergoing fully myeloablative HSPC transplantation, especially with sources of cells such as cord blood which typically engraft more slowly, as well as patients requiring repeated transfusion therapy.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Catherine M. Coy for assistance with the western blotting, George E. Sale for interpretation of cytospins, and Ariad Pharmaceuticals for the CIDs AP20187 and AP1903.
This work was supported by the National Institutes of Health (grant RO1 DK74522 [C.A.B.], grant P30 DK56465 [B.T.-S.], and grant UO1 HL099993 [B.T.-S. and D.W.E.]), a grant from the Tietze Foundation (D.W.E.), and a grant from the American Cancer Society (117682-MRSG-09-268-01-CCE [C.P.M.]).
Authorship
Contribution: E.B., C.P.M., B.T.-S., C.A.B., and D.W.E. designed experiments and analyzed data; E.B., C.P.M., A.N.K., and D.W.E. performed experiments; E.B., C.P.M., and D.W.E. prepared the figures; and E.B. and D.W.E. wrote the manuscript.
Conflict-of-interest disclosure: C.A.B. is named as an inventor on a patent for the CGS technology licensed to Ariad Pharmaceuticals Inc through the University of Washington. The remaining authors declare no competing financial interests.
Correspondence: David W. Emery, Institute for Stem Cell and Regenerative Medicine, University of Washington, 850 Republican St, Box 358056, Seattle, WA 98109; e-mail demery@uw.edu.