Key Points
CH50 activity reflects C5 blockade in PNH patients treated with eculizumab and is directly related to circulating free eculizumab levels.
Both CH50 and free eculizumab level markers look promising for the monitoring of complement blockade in patients with PNH receiving eculizumab.
Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by intravascular hemolysis, which is effectively controlled with eculizumab, a humanized monoclonal antibody that binds complement protein 5 (C5). The residual functional activity of C5 can be screened using a 50% hemolytic complement (CH50) assay, which is sensitive to the reduction, absence, and/or inactivity of any components of the classical and terminal complement pathway. Little data exist on complement blockade during treatment. From 2010 to 2012, clinical data, hemolysis biomarkers, complement assessment, and free eculizumab circulating levels were systematically measured immediately before every injection given to 22 patients with hemolytic PNH while receiving eculizumab therapy. During the study, 6 patients received ≥1 red blood cell transfusion. Lack of detectable CH50 activity (defined by CH50 ≤ 10% of normal values) was found in 184 samples (51%) and was significantly associated with lower lactate dehydrogenase levels (P = .002). Low levels of circulating free eculizumab (<50 µg/mL) correlated with detectable CH50 activity (CH50 > 10%; P = .004), elevated bilirubin levels (P < .0001), and the need for transfusions (P = .034). This study suggests that both CH50 activity and circulating free eculizumab levels may help physicians to manage PNH patients receiving eculizumab.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired clonal disorder of hematopoietic stem cells in which the lack of glycosylphosphatidylinositol-anchored protein complement-regulatory proteins (CD55 and CD59) leads to intravascular hemolysis.1-3 Eculizumab is a humanized monoclonal antibody that binds to complement protein 5 (C5), thereby inhibiting the formation of the terminal components of the complement cascade.4 Eculizumab has demonstrated reduced hemolysis, more stable hemoglobin levels, lower transfusion requirements, and improved quality of life,5-7 as well as reduced frequency of thromboembolic events,8 leading to an improvement in overall survival.9
However, 25% to 35% of patients are still transfused while undergoing treatment6,7,9-11 for a variety of reasons. These include breakthrough hemolysis caused by infections, surgery, or pregnancy, as well as underlying bone marrow failure or extravascular hemolysis.12,13 Kidney failure has also been reported in PNH,14 as well as iron deficiency,15 that might also impact the response to eculizumab. Recommended eculizumab doses and intervals have been designed to maintain a permanent complement blockade during the whole interdose period,5,6 with no consideration for adaptation from one patient to another. Although eculizumab directly targets the complement cascade, little data exist at present in the literature on long-term measures of complement activity during treatment,16 and no study to date has aimed to correlate clinical outcomes, terminal complement blockage activity, and free eculizumab levels.
The C5 can be screened using a routine 50% hemolytic complement (CH50) assay, which tests the functional activity of the classical and terminal complement pathways17 and is sensitive to the reduction, absence, and/or inactivity of any components of these 2 pathways. Our hypothesis was that this simple complement activity assay might help to manage patients with PNH on a consistent care basis. We therefore systematically measured complement activity and eculizumab dosage over an 18-month period in 22 PNH patients treated in our department and analyzed the relationship with respect to biological and clinical outcomes.
Material and methods
Patients
From October 2010 to February 2012, all patients with hemolytic PNH treated with eculizumab were prospectively followed systematically every 2 weeks at our institution. Patients with evidence of bone marrow failure at the time of eculizumab initiation (reticulocytes <60 × 109/L, platelets <100 × 109/L, and neutrophils <1.5 × 109/L) were systematically excluded, as this condition can affect the clinical response to eculizumab, regardless of how well hemolysis is controlled. All patients included in the study had a ≥6-month follow-up during treatment, because it has been shown that the response to eculizumab may improve during the first 6 months of treatment.16 The study was approved by the local ethic board, and written informed consent was obtained from all patients before samples were taken in accordance with the Declaration of Helsinki.
Study design
All patients received eculizumab intravenously (900 mg every 14 ± 2 days), as recommended.6 Clinical data (abdominal pain, hemoglobinuria, thrombosis events, and transfusion requirements); complete blood count; lactate dehydrogenase (LDH), haptoglobin, and bilirubin levels; and reticulocyte count were systematically collected immediately before every injection of eculizumab.
Complement assessment.
CH50 activity, C3d deposition, and C3 and C4 circulating levels were measured concomitantly to clinical and biological data immediately before every eculizumab injection. Peripheral blood samples were collected by venipuncture according to the standard procedure in EDTA, sent at +4°C within 3 hours to the laboratory, and then centrifuged (1000g for 10 minutes) to obtain plasma-EDTA depleted from platelets. Plasma samples were aliquoted in 0.5-mL volumes in cryovials and stored at −80°C. The CH50 assay was adapted from Kabat and Mayer’s method.17 In summary, this assay involves determining the quantity of plasma needed to induce 50% lysis of sheep erythrocytes sensitized with antibody anti-sheep erythrocytes (SRBCs). When SRBCs are incubated with test plasma, the classical complement pathway is activated, leading to the formation of a membrane attack complex and SRBC hemolysis. Plasma of patients was first prediluted in veronal buffer saline (VBS) at either 1 to 100 (standard dilution used for the determination of CH50 in patients with a normal or a subnormal level) or 1 to 31 (dilution for low CH50 activity such as patients with complement deficiency or under eculizumab therapy). Six different volumes (150, 200, 250, 300, 350, and 400 µL) of prediluted plasma of patients in VBS for a final volume of 800 µL were then incubated with a fixed volume of 200 µL SRBCs at 108/mL in VBS with MgCl2 and CaCl2 for 45 minutes at 37°C. The reaction was then stopped by adding saline solution, and the degree of hemolysis was quantified by measuring the absorbance of the hemoglobin released into the supernatant by spectrophotometry with a wavelength of 414 nm (protocol available on request). A reference plasma pool with a 100% CH50 arbitrary stored at −80°C is used as internal reference standard and incorporated in every subsequent assay. CH50 < 10% means that the tested plasma sample has <10% complement activity relatively to a pool of plasma from healthy donors (ie, CH50 = 100%). A representative experiment of lysis induced by a plasma pool (100%), plasma from a patient at 75%, and plasma from a hereditary C5-deficient patient (CH50 < 10%) is shown in supplemental Figure 1A-B, available on the Blood Web site. Residual complement activity is complement mediated using this assay as shown with plasma from a PNH patient receiving eculizumab with detectable CH50 activity at day 14 (patient 12, CH50 = 21%; supplemental Figure 1B). No lysis occurs in the presence of inactivated plasma (30 minutes at 56°C) or on dilution of the plasma, either with EGTA or plasma from a hereditary C5-deficient patient. We therefore define blocked CH50 (≤ 1%) that reflects the presence of <5% of functional C5 (supplemental Figure 1C) or unblocked CH50 (>10%). Thereafter, all the results are reported as a percentage of mean normal serum activity.
C3d deposition on red blood cells was quantified using flow cytometry (monoclonal anti-human C3d, clone A702; anti-human C3c, clone A701; and anti-human C3bi biotinylated, clone A710; Quidel; supplemental Figure 2).
Circulating levels of C3 and C4 were also studied by nephelometry, according to the instructions of the manufacturer (Siemens).
Free eculizumab dosage.
The plasma level of free eculizumab was assessed immediately prior to reinjection using a homemade enzyme-linked immunosorbent assay. Briefly, plastic plates were coated with C5 in phosphate-buffered saline (PBS; 10 µg/mL; Calbiochem) overnight at 4°C. Free sites were blocked with PBS containing 1.0% bovine serum albumin for 1 hour at 37°C. After washing, the plates were interacted with test plasmas diluted 1:4000 in PBS/bovine serum albumin containing 0.05% Tween 20 for 90 minutes at 37°C. Samples were tested in duplicate. Bound eculizumab was revealed using peroxidase-labeled goat anti-human IgG, followed by orthophenylenediamine. Normal plasma with a fixed concentration of eculizumab was used for the standard curve.
Statistical analysis
All data are presented, but only data recorded after ≥6 months of eculizumab treatment were used to study the association between free eculizumab levels or complement blockade activity and outcomes, to ensure that patients were assessed in a stable status. In our first analysis, the association of CH50 activity with biological parameters (LDH, hemoglobin, and bilirubin levels; reticulocyte count) was analyzed using linear mixed models, with random effects at patient level, including an intercept and a slope for duration of eculizumab treatment. This allowed for the correlation between measurements performed on the same patients to be taken into account, along with the evolution of parameters according to treatment duration. The association of nonblocked CH50 with clinical outcomes (thrombosis, abdominal pain, hemoglobinuria, and infection between eculizumab injections, as well as the need for transfusion after the current CH50 assessment) was analyzed using generalized linear models with logistic link function, with the same random effect structure as previously described. Similar methodology was used to analyze the association of C3 deposition and free eculizumab with biological and clinical parameters. In our second analysis, we used longitudinal Tobit regression models.18 These models account for the lower detection limit of 10% of CH50 measurements, as detailed in the supplemental Appendix, and for the correlation between measurements performed on the same patient. In this latter model, the dependent variable was CH50 activity. Models were analyzed within a Bayesian framework using Markov chain Monte Carlo methods implemented in BRugs.19
All tests were 2 sided, and P ≤ .05 was considered as indicating a significant association. Analyses were performed using the R statistical software version 2.15.0.20
Results
Patients
Twenty-two patients with hemolytic PNH treated with eculizumab were eligible for this study (7 males; median age, 42 years [range, 21-72 years]). Patient characteristics are described in Table 1. Before starting eculizumab, 21 patients were transfusion dependent, and 9 patients had a significant PNH-related complication (9 with thrombosis and 7 with previous history of aplastic anemia). During the study period, 6 patients received ≥1 transfusion (mean of 3 RBC transfusions per year), 2 patients developed deep vein thrombosis while receiving treatment, and 7 patients were diagnosed with ≥1 upper tract respiratory infection episode. None of the patients had surgery or were pregnant during the study period.
Patient characteristics
| UPN . | PNH type . | TE . | Tx/year . | Hb . | ARC . | LDH . | T bili . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | During . | Before . | During . | Before . | During . | Before . | During . | Before . | During . | Before . | During . | ||
| 3 | AA-PNH | BC, DVT | No | 6 | 0 | 8.9 | 10.2 | 123 | 102 | 4000 | 397 | 45 | 17 |
| 11 | C-PNH | BC | No | 6 | 0 | 6 | 12.5 | 200 | 147 | 1500 | 478 | 40 | 20.2 |
| 18 | C-PNH | No | No | 1 | 0 | 9.9 | 11.1 | 80 | 121 | 2108 | 411 | 11 | 17 |
| 5 | AA-PNH | BC | No | 4 | 0 | 8.9 | 11.4 | 130 | 158 | 1765 | 487 | 12 | 19 |
| 20 | C-PNH | DVT, PE | No | 2 | 0 | 10.2 | 10.6 | 95 | 78 | 2439 | 571 | 9 | 13.8 |
| 9 | AA-PNH | No | No | 12 | 0 | 8.7 | 11.6 | 241 | 246 | 3000 | 353 | 25 | 11.3 |
| 13 | C-PNH | No | DVT | 0 | 0 | 9.2 | 9.9 | 73 | 95 | 3000 | 492 | 30 | 23 |
| 10 | AA-PNH | No | No | 6 | 0 | 7.2 | 9.3 | 33 | 195 | 2000 | 354 | 62 | 30.2 |
| 2 | C-PNH | No | No | 6 | 0 | 7 | 10.9 | 200 | 282 | 2500 | 623 | 45 | 26.4 |
| 12 | Int-PNH | No | No | 6 | 0 | 8 | 9.9 | 172 | 191 | 1000 | 467 | 21 | 36.1 |
| 14 | C-PNH | No | No | 2 | 0 | 8.5 | 10.1 | 150 | 102 | 3500 | 450 | 21 | 13.9 |
| 16 | C-PNH | No | No | 6 | 0 | 8.5 | 8.9 | 200 | 257 | 2100 | 421 | 11 | 33 |
| 17 | C-PNH | MVT | No | 6 | 0 | 9 | 9.9 | 150 | 139 | 3553 | 553 | 12 | 10.8 |
| 8 | C-PNH | DVT | No | 4 | 0 | 8.3 | 9.6 | 250 | 184 | 3700 | 565 | 9.7 | 17.9 |
| 22 | AA-PNH | BC | No | 6 | 0 | 9 | 10 | 170 | 202 | 800 | 299 | 8 | 71 |
| 21 | AA-PNH | No | No | 1 | 0 | 9.1 | 9.6 | 85 | 52 | 1925 | 621 | 12.8 | 11.9 |
| 1 | C-PNH | No | DVT | 12 | 0.8 | 7.5 | 8.8 | 160 | 145 | 1500 | 453 | 24 | 21.8 |
| 4 | C-PNH | BC | No | 6 | 0.6 | 7 | 10.2 | 150 | 120 | 2000 | 465 | 25 | 17.3 |
| 7 | AA-PNH | No | No | 36 | 0.8 | 5.9 | 9.7 | 350 | 240 | 2100 | 362 | 20 | 15.5 |
| 15 | C-PNH | No | No | 12 | 0.8 | 10.6 | 10.2 | 205 | 235 | 3000 | 515 | 16 | 17.4 |
| 19 | C-PNH | DVT, PE | No | 6 | 5.8 | 5 | 8.7 | 160 | 161 | 2000 | 507 | 10 | 16.7 |
| 6 | C-PNH | No | No | 8 | 1.1 | 7.7 | 8.2 | 175 | 352 | 1000 | 865 | 9 | 27 |
| UPN . | PNH type . | TE . | Tx/year . | Hb . | ARC . | LDH . | T bili . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | During . | Before . | During . | Before . | During . | Before . | During . | Before . | During . | Before . | During . | ||
| 3 | AA-PNH | BC, DVT | No | 6 | 0 | 8.9 | 10.2 | 123 | 102 | 4000 | 397 | 45 | 17 |
| 11 | C-PNH | BC | No | 6 | 0 | 6 | 12.5 | 200 | 147 | 1500 | 478 | 40 | 20.2 |
| 18 | C-PNH | No | No | 1 | 0 | 9.9 | 11.1 | 80 | 121 | 2108 | 411 | 11 | 17 |
| 5 | AA-PNH | BC | No | 4 | 0 | 8.9 | 11.4 | 130 | 158 | 1765 | 487 | 12 | 19 |
| 20 | C-PNH | DVT, PE | No | 2 | 0 | 10.2 | 10.6 | 95 | 78 | 2439 | 571 | 9 | 13.8 |
| 9 | AA-PNH | No | No | 12 | 0 | 8.7 | 11.6 | 241 | 246 | 3000 | 353 | 25 | 11.3 |
| 13 | C-PNH | No | DVT | 0 | 0 | 9.2 | 9.9 | 73 | 95 | 3000 | 492 | 30 | 23 |
| 10 | AA-PNH | No | No | 6 | 0 | 7.2 | 9.3 | 33 | 195 | 2000 | 354 | 62 | 30.2 |
| 2 | C-PNH | No | No | 6 | 0 | 7 | 10.9 | 200 | 282 | 2500 | 623 | 45 | 26.4 |
| 12 | Int-PNH | No | No | 6 | 0 | 8 | 9.9 | 172 | 191 | 1000 | 467 | 21 | 36.1 |
| 14 | C-PNH | No | No | 2 | 0 | 8.5 | 10.1 | 150 | 102 | 3500 | 450 | 21 | 13.9 |
| 16 | C-PNH | No | No | 6 | 0 | 8.5 | 8.9 | 200 | 257 | 2100 | 421 | 11 | 33 |
| 17 | C-PNH | MVT | No | 6 | 0 | 9 | 9.9 | 150 | 139 | 3553 | 553 | 12 | 10.8 |
| 8 | C-PNH | DVT | No | 4 | 0 | 8.3 | 9.6 | 250 | 184 | 3700 | 565 | 9.7 | 17.9 |
| 22 | AA-PNH | BC | No | 6 | 0 | 9 | 10 | 170 | 202 | 800 | 299 | 8 | 71 |
| 21 | AA-PNH | No | No | 1 | 0 | 9.1 | 9.6 | 85 | 52 | 1925 | 621 | 12.8 | 11.9 |
| 1 | C-PNH | No | DVT | 12 | 0.8 | 7.5 | 8.8 | 160 | 145 | 1500 | 453 | 24 | 21.8 |
| 4 | C-PNH | BC | No | 6 | 0.6 | 7 | 10.2 | 150 | 120 | 2000 | 465 | 25 | 17.3 |
| 7 | AA-PNH | No | No | 36 | 0.8 | 5.9 | 9.7 | 350 | 240 | 2100 | 362 | 20 | 15.5 |
| 15 | C-PNH | No | No | 12 | 0.8 | 10.6 | 10.2 | 205 | 235 | 3000 | 515 | 16 | 17.4 |
| 19 | C-PNH | DVT, PE | No | 6 | 5.8 | 5 | 8.7 | 160 | 161 | 2000 | 507 | 10 | 16.7 |
| 6 | C-PNH | No | No | 8 | 1.1 | 7.7 | 8.2 | 175 | 352 | 1000 | 865 | 9 | 27 |
AA-PNH, aplastic anemia-PNH; ARC, absolute reticulocyte count (×109); BC, Budd-Chiari syndrome; C-PNH, classic PNH; DVT, deep vein thrombosis; Hb, hemoglobin level; Int-PNH, intermediate PNH; MVT, mesenteric vein thrombosis; PE, pulmonary embolism; T bili, unfractionated bilirubin (μM/L); TE, thromboembolic events; Tx/year, number of packed red blood cell units transfused in the last year (before) or in 1 year during eculizumab treatment (during); UPN, unique patient number.
CH50 assessment after in vitro addition of eculizumab to plasma from healthy individuals and from PNH patients not receiving eculizumab
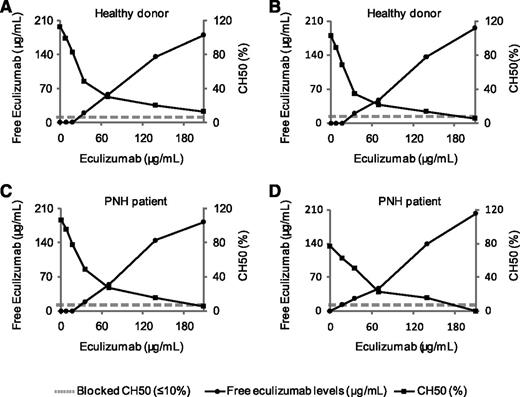
We first assessed the dose-response curve of eculizumab on CH50 in plasma from healthy individuals and from PNH patients to confirm the relationship between higher doses of eculizumab and the inhibition of CH50 in the setting of PNH. Increasing amounts of eculizumab (up to 210 μg/mL) were added to the plasma of 2 healthy donors and 2 hemolytic PNH patients not receiving eculizumab. CH50 and free eculizumab levels were measured. As shown in Figure 1, increasing amounts of eculizumab induce a decrease of CH50 activity. Lack of detectable CH50 activity (<10%) was obtained after in vitro addition of 210 μg/mL of eculizumab for the 4 tested plasma samples. Free eculizumab levels were detectable after the addition of 30 μg/mL. Dose-response curves were comparable between healthy controls and PNH patients.
CH50 and free eculizumab levels after in vitro addition of eculizumab to plasma from 2 healthy individuals and 2 untreated PNH patients. CH50 (•) and free eculizumab levels (♦) are shown for each time point. (A-B) Healthy controls. (C-D) Hemolytic PNH patients not receiving eculizumab.
CH50 and free eculizumab levels after in vitro addition of eculizumab to plasma from 2 healthy individuals and 2 untreated PNH patients. CH50 (•) and free eculizumab levels (♦) are shown for each time point. (A-B) Healthy controls. (C-D) Hemolytic PNH patients not receiving eculizumab.
In vivo association between CH50 activity and residual intravascular hemolysis
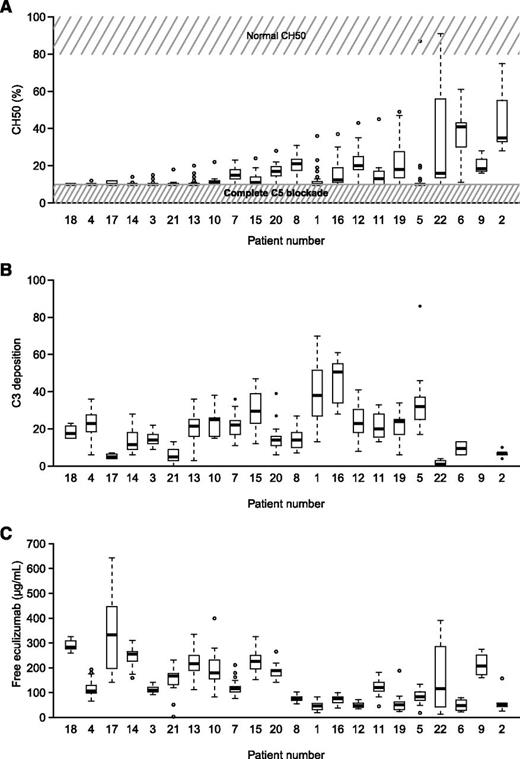
To investigate whether the residual intravascular hemolysis present in certain eculizumab-treated PNH patients could be explained by an incomplete C5 blockage, CH50 activity was systematically assessed immediately prior to eculizumab reinjection. With a median follow-up from first sampling of 13 months (interquartile range, 10-15 months), 364 samples were obtained after a minimum of 6 months of eculizumab treatment (median, 18 per patient; range, 1-31 per patient). Detectable hemolytic activity (CH50 > 10%) was present in 49% of our patient samples. CH50 measurements in all patients are shown in Figure 2A.
CH50 activity, C3d deposition, and free eculizumab dosage in PNH patients receiving eculizumab. (A) Box plot showing CH50 activity in all patients. Normal CH50 value and complete C5 blockade are represented in gray. (B) C3d deposition in all patients during the entire study period. (C) Box plot showing free eculizumab dosage in all patients. Box and whisker plots display the median and 25th and 75th percentiles of the distribution (box). Whiskers extend to the most extreme data point, no more than 1.5 times the interquartile range from the box. During the study period, patients 1 and 13 experienced thrombosis, whereas patients 1, 4, 6, 7, 15, and 19 were transfused at least once.
CH50 activity, C3d deposition, and free eculizumab dosage in PNH patients receiving eculizumab. (A) Box plot showing CH50 activity in all patients. Normal CH50 value and complete C5 blockade are represented in gray. (B) C3d deposition in all patients during the entire study period. (C) Box plot showing free eculizumab dosage in all patients. Box and whisker plots display the median and 25th and 75th percentiles of the distribution (box). Whiskers extend to the most extreme data point, no more than 1.5 times the interquartile range from the box. During the study period, patients 1 and 13 experienced thrombosis, whereas patients 1, 4, 6, 7, 15, and 19 were transfused at least once.
We then analyzed the relationship between C5 blockage and biological and clinical outcomes. Of the 364 samples, a lack of detectable CH50 (CH50 ≤ 10%) was found in 184 (51%) samples. Blocked CH50 was significantly associated with lower LDH levels (P = .002; supplemental Figure 3). We confirmed this statistically significant association between blocked CH50 and lower LDH levels using longitudinal Tobit regression models, which allowed us to take into account the lowest possible detection limit (10%) of the technique for CH50 activity. On average, we observed an increase in CH50 of 1.4% per 100 IU/L increase in LDH (95% confidence interval of 0.03-2.7). We also observed lower hemoglobin levels, higher bilirubin levels, and higher reticulocyte counts in cases of incomplete blockage (CH50 > 10%), although no difference was statistically significant (supplemental Figure 3). Moreover, no differences in the rates of abdominal pain or hemoglobinuria, infection, or the need for transfusion were found (P = .51, P = .82, P = .15, and P = .68, respectively). Two patients presented with thrombosis at 1 visit, with a concomitant CH50 of 10% and 13%, respectively.
Alternative pathway activation
To investigate the influence of fluid-phase complement activation, C3 and C4 circulating levels were systematically analyzed at the same time point as previously (immediately prior to injection). C3 and C4 levels were in the normal range during the entire study period in all but 1 patient (patient 15), who presented a low C3 level (394 mg/L; the normal is between 660 and 1250 mg/L) and a normal C4 level, suggesting alternative pathway activation.
C3d deposition
It has recently been suggested that the C5 blockade enables C3 fragment accumulation on certain PNH red blood cells, which might serve to explain the residual low-level hemolysis occurring in certain eculizumab-treated patients. We therefore assessed type III PNH red blood cell C3 deposition (in all but 1 patient; 277 samples; median 14 per patient; range, 2-21). As expected,12,13 analysis of red blood cells from PNH patients receiving eculizumab showed a distinct population of C3d-positive cells (supplemental Figure 2). We detected both a significant inter- and intrapatient variability over time (Figure 2B). Moreover, we found no correlation between C3 opsonization and clinical or biological signs of hemolysis (data not shown).
Complement activity and free eculizumab dosage
Free eculizumab dosage is represented in Figure 2C for all patients during the entire study period. With the same schedule of 900 mg every 2 weeks, residual levels of free eculizumab varied from 18 to 643 µg/mL, with a certain amount of intrapatient variability over time in several patients.
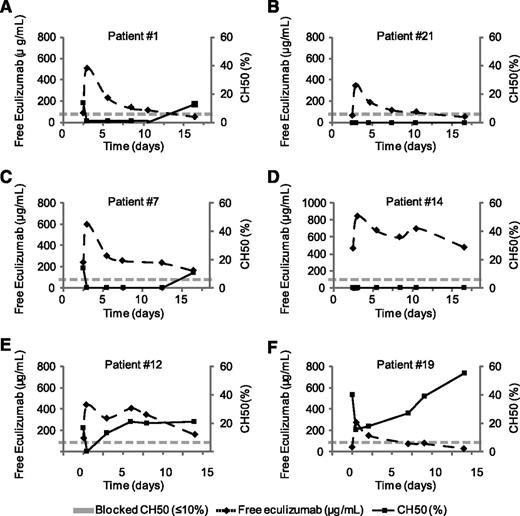
To examine more precisely the relationship between CH50 and eculizumab levels, we analyzed both markers sequentially in 6 patients receiving treatment 1 hour, 3 days, 6 days, and 9 days after injection of eculizumab and immediately prior to the next injection (day 14 in most cases). As shown in Figure 3A-F, we found an inverse correlation between both markers in all patients. Different pharmacokinetic profiles with T1/2 values spanning from 4 to 21 days were obtained.
Kinetics of CH50 and free eculizumab levels in PNH patients following eculizumab injection. Plasma from 6 PNH patients receiving eculizumab were drawn before eculizumab injection, 1 hour, 3 days, 6 days, and 9 days after injection of eculizumab, and immediately prior to the next injection (day 14 in most cases). Dose-response curves are represented using CH50 (left y-axis) and levels of free eculizumab (right y-axis) by timing after injection (days, x-axis). Half-life (T1/2) was 4.3 days for (A) patient 1, (B) 5.7 days for patient 21, (C) 8.6 days for patient 7, (D) 20.3 days for patient 14, (E) 11.2 days for patient 12, and (F) 5 days for patient 19.
Kinetics of CH50 and free eculizumab levels in PNH patients following eculizumab injection. Plasma from 6 PNH patients receiving eculizumab were drawn before eculizumab injection, 1 hour, 3 days, 6 days, and 9 days after injection of eculizumab, and immediately prior to the next injection (day 14 in most cases). Dose-response curves are represented using CH50 (left y-axis) and levels of free eculizumab (right y-axis) by timing after injection (days, x-axis). Half-life (T1/2) was 4.3 days for (A) patient 1, (B) 5.7 days for patient 21, (C) 8.6 days for patient 7, (D) 20.3 days for patient 14, (E) 11.2 days for patient 12, and (F) 5 days for patient 19.
We then analyzed the correlation between CH50 blockage and the residual level of free eculizumab immediately prior to reinjection. As shown in supplemental Figure 4, there was a strong correlation between a higher residual level of free eculizumab and CH50 blockage (P = .002). We completed our analysis using a longitudinal Tobit model to take into account the detection limit of CH50 values of 10% or lower. The relationship yielded a significant association between both markers. The slope was −0.08 (95% credibility interval of −0.15 to −0.02), thus confirming that as free eculizumab levels rise, the percentage of CH50 decreases.
We then hypothesized that the level of circulating free eculizumab immediately prior to reinjection might be associated with clinical and biological outcomes. The results are presented in Table 2. Analysis using mixed models, with random patients, and adjustment for treatment duration, identified the following variables as being associated with lower levels of residual free eculizumab: elevated bilirubin (P < .0001), need for transfusion (P = .036), and nonblocked CH50 (P = .004). Of note, LDH and hemoglobin levels and the reticulocyte count were not associated with residual free eculizumab levels. Regarding clinical outcomes, lower free eculizumab levels were associated with higher rates of hemoglobinuria (P = .030). No significant difference was found for abdominal pain and infection (P = .098 and P = .30, respectively).
Correlation between circulating levels of eculizumab, CH50, and outcomes in PNH patients treated with eculizumab
| Variables . | Eculizumab trough level . | |||
|---|---|---|---|---|
| <35 µg/mL . | 35-100 µg/mL . | 100-200 µg/mL . | >200 µg/mL . | |
| Number of samples | 19 | 140 | 124 | 70 |
| LDH, median (IQR) IU/L | 459 (402-516) | 462 (428-520) | 450 (377-492) | 467 (435-504) |
| N (%) >1.5 ULN | 3 (18) | 9 (7) | 6 (6) | 1 (2) |
| Hemoglobin, median (IQR) g/dL | 9.1 (8.7-9.4) | 9.6 (9.0-10.6) | 10.1 (9.8-10.8) | 10.3 (9.9-10.6) |
| Bilirubin, median (IQR) µM/L | 24 (21-26) | 22 (17-31) | 18 (15-20) | 17 (14-24) |
| ARC, median (IQR) ×103/L | 148 (139-165) | 181 (148-227) | 139 (112-198) | 110 (98-212) |
| Need for transfusion, N (%) | 2 (11) | 2 (1) | 1 (1) | 0 (0) |
| Blocked CH50, N (%) | 6 (32) | 55 (39) | 67 (54) | 47 (68) |
| Hemoglobinuria, N (%) | 5 (26) | 33 (24) | 27 (22) | 7 (11) |
| Abdominal pain, N (%) | 5 (26) | 13 (9) | 20 (17) | 1 (2) |
| Infection, N (%) | 0 (0) | 6 (6) | 6 (6) | 4 (7) |
| Variables . | Eculizumab trough level . | |||
|---|---|---|---|---|
| <35 µg/mL . | 35-100 µg/mL . | 100-200 µg/mL . | >200 µg/mL . | |
| Number of samples | 19 | 140 | 124 | 70 |
| LDH, median (IQR) IU/L | 459 (402-516) | 462 (428-520) | 450 (377-492) | 467 (435-504) |
| N (%) >1.5 ULN | 3 (18) | 9 (7) | 6 (6) | 1 (2) |
| Hemoglobin, median (IQR) g/dL | 9.1 (8.7-9.4) | 9.6 (9.0-10.6) | 10.1 (9.8-10.8) | 10.3 (9.9-10.6) |
| Bilirubin, median (IQR) µM/L | 24 (21-26) | 22 (17-31) | 18 (15-20) | 17 (14-24) |
| ARC, median (IQR) ×103/L | 148 (139-165) | 181 (148-227) | 139 (112-198) | 110 (98-212) |
| Need for transfusion, N (%) | 2 (11) | 2 (1) | 1 (1) | 0 (0) |
| Blocked CH50, N (%) | 6 (32) | 55 (39) | 67 (54) | 47 (68) |
| Hemoglobinuria, N (%) | 5 (26) | 33 (24) | 27 (22) | 7 (11) |
| Abdominal pain, N (%) | 5 (26) | 13 (9) | 20 (17) | 1 (2) |
| Infection, N (%) | 0 (0) | 6 (6) | 6 (6) | 4 (7) |
Two case reports where assessment of both CH50 and residual free eculizumab were helpful in managing dose adjustments in PNH patients receiving eculizumab.
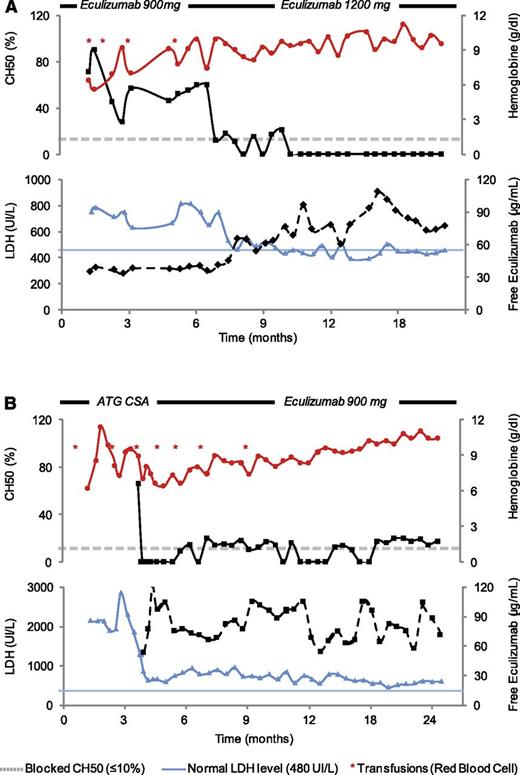
A 63-year-old man was referred to our center because of hemolytic PNH with no peripheral sign of bone marrow failure (neutrophils and platelets were normal). The patient received red blood cell transfusions every 2 weeks because of hemolysis, which justified the introduction of eculizumab. As shown in Figure 4A, the patient was still transfused every 3 weeks after 6 months of treatment. The particularity of this patient was a body mass index of 29. LDH was less than twice the normal level. An assessment of complement activity showed CH50 values that regularly surpassed 40% and free eculizumab levels <50 μg/mL prior to reinjection. Because of these reasons, we increased the eculizumab dose to 1200 mg every 15 days, which dramatically improved the hematologic condition of this patient, who became transfusion independent with a CH50 blocked (<10%) 3 months later (Figure 4A).
Monitoring of free eculizumab levels and CH50 in 2 representative cases. (A) Overweight patient. (B) Aplastic PNH syndrome. For each panel, hemoglobin (red filled circles), CH50 (black filled square with dash line), LDH (blue filled triangle), and free eculizumab (black filled squares with solid line) levels are shown. A gray dash line indicates blocked CH50 (≤10%), whereas a blue dash line represents the normal value for LDH (upper limit, 480 UI/L). Red asterisks represent red blood cell transfusion episodes during follow-up. Treatment and eculizumab doses during follow-up are indicated.
Monitoring of free eculizumab levels and CH50 in 2 representative cases. (A) Overweight patient. (B) Aplastic PNH syndrome. For each panel, hemoglobin (red filled circles), CH50 (black filled square with dash line), LDH (blue filled triangle), and free eculizumab (black filled squares with solid line) levels are shown. A gray dash line indicates blocked CH50 (≤10%), whereas a blue dash line represents the normal value for LDH (upper limit, 480 UI/L). Red asterisks represent red blood cell transfusion episodes during follow-up. Treatment and eculizumab doses during follow-up are indicated.
The second patient was a 24-year-old man who was referred to our center because of moderate aplastic anemia (neutrophils, 1000 G/L; reticulocytes, 35 000 G/L; hemoglobin level, 7.5 g/dL; platelets, 10 000 G/L) with hemolysis (LDH level 1000 IU/L) which revealed a PNH clone (clone size: 65%). Because of weekly platelet transfusions, the patient was first given immunosuppressive treatment (association of horse anti-thymocyte globulin and cyclosporine) followed by eculizumab 3 months later due to persistence signs of hemolysis and low hemoglobin levels. Three months after eculizumab initiation, the patient continued to present high LDH levels. An assessment of complement activity showed CH50 values that were regularly <15% prior to reinjection and free eculizumab levels >50 μg/mL. We therefore decided not to increase the eculizumab dose, attributing the LDH levels to the hematopoiesis recovery in the context of aplastic anemia recently treated with immunosuppressive treatment. Three months later, the patient recovered normal levels of both hemoglobin and LDH (Figure 4B).
Discussion
Our study shows that detectable CH50 activity is a simple biomarker directly related to both intravascular hemolysis (LDH levels) and circulating free eculizumab levels in patients with hemolytic PNH during treatment. We found a correlation between in vitro CH50 and free eculizumab levels in sequential sample analysis of PNH patients receiving treatment. We eventually showed that both markers look promising for the monitoring of complement blockade in patients with PNH under eculizumab and may eventually help physicians to manage PNH patients receiving treatment. Two representative case reports are presented above. Conversely, another study finding is that C3d deposition on glycosylphosphatidylinositol-anchored protein–negative red blood cells did not have an impact on hematologic outcomes in our cohort of patients, who were prospectively and sequentially followed during an 18-month period.
To the best of our knowledge, this is the first large pharmacodynamics study on eculizumab. Our main finding is that detectable CH50 activity is associated with residual intravascular hemolysis (LDH levels) in patients receiving eculizumab. One may argue against our ability to firmly establish a correlation between CH50 activity and clinical outcomes, such as hemoglobin level and/or the need for transfusions. However, patients with nonblocked CH50 had a trend for lower hemoglobin levels, whereas only 6 of 22 patients were still being transfused and receiving treatment during the follow-up period. The pharmacodynamics of eculizumab were assessed in only a few reported series. A hemolytic assay measuring the capacity of patient serum to lyse chicken erythrocytes has previously been used.5 The hemolytic activity of patient serum was completely blocked during the entire study period, with a limit of <20% using a chicken red blood cells assay. In our study, residual patient plasma hemolytic activity was assessed through a quantitative CH50 assay using sensitized sheep red blood cells. In this setting, 1% of C7 reestablished detectable CH50 activity (23% of normal values)21 and <5% of functional C5 reestablished detectable CH50 activity (supplemental Figure 1C). However, the result with CH50 is an average relationship and patient variability was high so that no threshold with fair discriminative properties can be identified at an individual level. Moreover, it is not technically possible using this assay to define precisely the level of CH50 below the detection limit of 10%. Linear correlations are therefore impossible to calculate. Blocked vs unblocked CH50 was defined accordingly (≤10%; also seen in hereditary terminal pathway deficiency vs >10%), and we used a longitudinal Tobit regression model that accounts both for repeated measurements and the lower 10% limit of CH50 detection to confirm our findings.
Detectable hemolytic activity (CH50 > 10%) was present in 49% of our patient samples related to a functional amount of C5 in the circulation, which was associated with significantly higher LDH levels. This may suggest that residual hemolysis may be related to incomplete C5 blockage. CH50 activity was also closely related to circulating levels of eculizumab. Of the 195 patients who participated in 1 of the 3 prospective parent trials,5-7 trough concentrations of eculizumab level <35 µg/mL were observed in 49 of 135 patients (36.3%) following the first dose of eculizumab,16 establishing a clear relationship with higher rates of intravascular hemolysis (36 of these 49 patients exhibited hemolysis, with a CH50 activity >20%). In our study, residual free eculizumab was >35 µg/mL in 95% of the tested samples (334 of 353 samples; Table 2), displaying a clear relationship with clinical outcomes (significantly fewer transfusions). Multiple occurrences of low eculizumab levels (defined as a concentration of free eculizumab <50 µg/mL) were observed in roughly 16% of tested samples, which is in accordance with the frequency of underdosing eculizumab in reported patients (10%)22 and a higher risk to develop hemolytic events during treatment. The standard eculizumab maintenance dose of 900 mg every 14 days is sufficient to maintain inhibition of hemolysis in most patients.16 However, a number of patients did report breakthrough hemolysis and required the dosing interval to be shortened to <14 days or for every single dose to be increased. In our experience, all samples with free eculizumab residual levels of 150 µg/L or more never experienced a breakthrough during the entire follow-up period.
Moreover, on a daily care basis, it is not always easy to identify patients where a modification of eculizumab schedule may be beneficial. LDH levels are not always useful in this situation. We report on 2 patients for whom the assessment of both markers facilitated in adjusting doses (overweight and aplastic anemia/PNH syndrome; Figure 4) and in which our evaluation led us to increase the dose in one case but to continue treatment with the same dose in the other. Our study also highlights the high heterogeneity of eculizumab’s pharmacodynamics in patients. The level of complement blockage is not strictly reproducible from one patient to another for the same circulating level of eculizumab. There are also some situations where there is a residual functional C5 activity in the presence of a large excess of free eculizumab for reasons that are still unknown. We did not find in our patients genetic variant in C5 recently published to be associated with poor response to eculizumab.23 Moreover, half-life was very heterogeneous in our patients, without any clear explanation as to why this should occur, because none of our patients presented kidney failure or any physical conditions that may explain such findings. We therefore feel that careful monitoring of C5 blockade using both a CH50 assay and assessing the eculizumab trough level may be helpful in evaluating possible underdosing, especially in specific situations including infections,24,25 pregnancy or postpartum events,26,27 surgery, and trauma28,29 that may augment the activation or production of complement factors, including C5. Our results also highlight that some patients may require a lower dosage and/or longer intervals between doses, which should only be explored through well-designed prospective controlled trials. For it is fundamentally important, as recently discussed for atypical hemolytic uremic syndrome patients, to identify the best dose and dose schedule for PNH patients receiving eculizumab due to the extremely high cost of this life-long treatment.30
Conversely, we did not find any correlation between the level of C3d opsonization and clinical or biological signs of hemolysis, whereas a rough correlation between C3 deposition and clinical response was previously found in 2 independent series of patients.12,13 In these latter studies, a small number of patients with a substantial proportion of C3-coated red blood cells became transfusion independent, whereas a number of patients with no C3d depositions still required transfusions. It is therefore possible that the small number of patients in our study is responsible for this finding. Moreover, C3 depositions may only acquire a role in clinical/hematologic outcome if downstream events (namely membrane attack complex formation) are fully inhibited, whereas we showed that residual terminal complement activity (as measured by CH50) remained in half of our patients, which may also explain the absence of relationship in our study. However, it is also surprising that subjects who are genetically deficient in CD55 (the so-called Inab phenotype) do not experience extravascular hemolysis. Given the dramatic reduction in transfusion requirements and the regular C3d depositions in almost all our patients, our data suggest that other factors are also involved in determining whether patients become transfusion independent. One possible explanation is individual genetic polymorphism in different complement regulators, such as factor H,31,32 factor I,33 complement receptor 1,34 and even glycophorin itself,35 that may be involved in the control of residual hemolysis. The association between allelic variants complement receptor 1 and transfusion dependence in patients receiving eculizumab has in fact been the subject of a recent publication.36
Our work has strengths and limitations. The strengths include the homogeneous cohort of PNH patients enrolled in our center with only hemolytic PNH (no patients with evidence of bone marrow failure), who were followed for >6 months after beginning eculizumab. However, our study is limited because of the relatively small number of patients and by the low number of clinical events observed during treatment.
In conclusion, our results suggest that CH50 activity is a simple biomarker directly related to both intravascular hemolysis (LDH levels) and circulating free eculizumab levels in patients with hemolytic PNH during treatment. We believe that a concomitant assessment of CH50 with free eculizumab levels look promising for the monitoring of complement blockade in patients with PNH under eculizumab and may eventually help physicians to manage such patients on a regular basis and to evaluate possible underdosing.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.P.d.L., V.F.-B., P.R.-O., and G.S. conceived and designed the study; R.P.d.L. and G.S. provided study materials and patients; R.P.d.L., V.F.-B., R.P., A.X., D.C.C., P.V.-M., J.R., P.R.-O., A.P., F.S.d.F., M.R., and G.S. provided data collection and assembly; R.P.d.L., V.F.-B., R.P., P.R.-O., and G.S. provided data analysis and interpretation; R.P.d.L., V.F.-B., R.P., and G.S. wrote the manuscript; and R.P.d.L., V.F.-B., R.P., A.X., P.V.-M., J.R., D.C.C., P.R.-O., A.P., F.S.d.F., M.R., S.A., S.R., and G.S. provided final approval of the manuscript.
Conflict-of-interest disclosure: R.P.d.L., G.S., and V.F.-B. are consultants for Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Régis Peffault de Latour, Service d’Hématologie Greffe, Hôpital Saint Louis, 75010 Paris, France; e-mail: regis.peffaultdelatour@sls.aphp.fr; and Véronique Frémeaux-Bacchi, Service d’Immunologie Biologique, Hôpital Européen Georges Pompidou, 20-40 rue Leblanc, 75908 Paris cedex 15, France; e-mail: veronique.fremeaux-bacchi@egp.aphp.fr.
References
Author notes
R.P.d.L. and V.F.-B. contributed equally to this work.