In this issue of Blood, Shah et al describe the onset of severe acute graft-versus-host disease (GVHD) in 5 of 9 patients who received infusions of ex vivo expanded donor natural killer (NK) cells after HLA-matched hematopoietic stem cell transplantation (HSCT).1
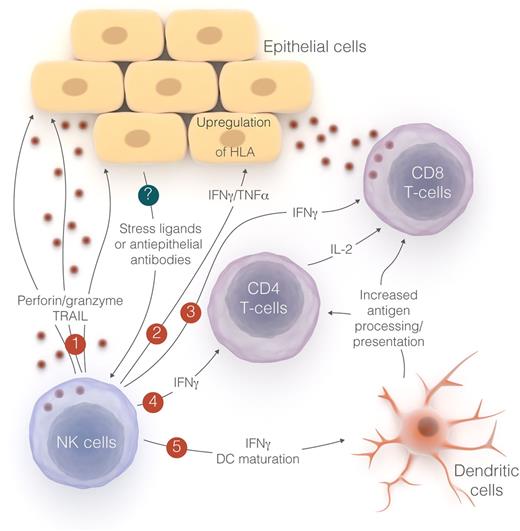
Possible mechanisms of NK cell–mediated acute GVHD. Subclinical GVHD triggers NK cell activation through unknown mechanisms (denoted by “?”), perhaps involving antiepithelial antibodies or expression of stress ligands induced by local inflammation. The resulting NK-cell responses may be targeted directly at epithelial cells (1), or may indirectly activate adaptive immune mechanisms that exacerbate T-cell–mediated GVHD (2-5). Professional illustration by Luk Cox.
Possible mechanisms of NK cell–mediated acute GVHD. Subclinical GVHD triggers NK cell activation through unknown mechanisms (denoted by “?”), perhaps involving antiepithelial antibodies or expression of stress ligands induced by local inflammation. The resulting NK-cell responses may be targeted directly at epithelial cells (1), or may indirectly activate adaptive immune mechanisms that exacerbate T-cell–mediated GVHD (2-5). Professional illustration by Luk Cox.
Despite conducting a small phase 1 trial with significant diversity in patients, donors, products, and treatment, the authors provide careful correlative studies that suggest exacerbation of subclinical T-cell–mediated skin and gut graft-versus-host as a possible mechanism. By carefully evaluating the manufacturing process, characteristics of the NK-cell and stem cell products, and patient pathology, the authors demonstrate a previously unrecognized potential for NK cells to have a profound effect in promoting GVHD in this context.
This clinical trial used CD34 selection and CD3 depletion of the starting apheresis product to deliver a very low dose of T cells in both the stem cell graft and NK cell product, enabling a posttransplant period without immune-suppressive drugs that would interfere with NK-cell function. T-cell doses were <2 × 104/kg in the stem cell graft and <2 × 103/kg in the NK-cell infusion. NK-cell doses were also very low compared with previously published trials; subjects received only 105 to 106/kg.
That NK cells can discriminate between healthy and malignant cells despite lacking antigen specificity, separating graft-versus-leukemia (GVL) from GVHD, is especially striking in the setting of HLA mismatch, where NK cells appear to have the most pronounced effect. More strikingly, NK cells may mediate a GVL effect while simultaneously mediating a decrease in GVHD, perhaps because missing self alone is insufficient, and normal tissues do not express the requisite activating ligands to induce an NK-cell response. Genetic determinants of NK-cell function may have value in predicting GVHD in the transplant setting,2,3 but rapid NK-cell reconstitution4 and higher NK-cell graft content5 are associated with decreased GVHD. Moreover, hundreds of patients treated in clinical trials infusing NK cells derived by apheresis and T-cell depletion have shown no infusion-related dose-limiting toxicity or evidence of GVHD exacerbation, whether delivered after high-dose chemotherapy6 or after mismatched HSCT.7 Thus, the GVHD seen in this report is quite unexpected.
Possible factors in the current report that may help explain the unexpected GVHD rates include the timing of NK-cell infusion (8-35 days after transplant), the lack of posttransplant immunosuppression, or the hyperactivation of the NK cells, which were expanded on interleukin 15-secreting feeder cells. It is important to note that all 4 patients receiving unrelated donor transplants developed GVHD compared with only 1 of 5 patients receiving related donor transplants, further implicating a T-cell etiology mediated by minor antigens that was somehow exacerbated by the infused NK cells.
This then raises several possible mechanisms to explain the observed GVHD. As shown in the figure, subclinical dermal or mucosal inflammation may increase stress ligands, rendering epithelium susceptible to recognition by NK cells, causing (1) a direct effect via lysis or indirect activation of adaptive immunity through (2) cytokine-mediated upregulation of HLA for T-cell recognition, (3) stimulation of cytotoxic T cells, (4) activation of helper T cells, or (5) maturation of dendritic cells for enhanced antigen presentation or crosspresentation.
Whichever the case, these findings force us to recognize the potential potency of NK cells and to consider that GVL is no longer discretely separated from GVHD for NK cells. Further understanding of this mechanism is essential for understanding GVHD and the future of adoptive cell therapy with NK cells.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal