In this issue of Blood, Stepensky et al provide an astute description of immunosenescence arising from deficiency in tripeptidyl peptidase II (TPPII).1 Senescence of T and B lymphocytes is a striking finding, which has recently come into the limelight because it can be linked to primary immunodeficiency syndromes with autoimmunity.
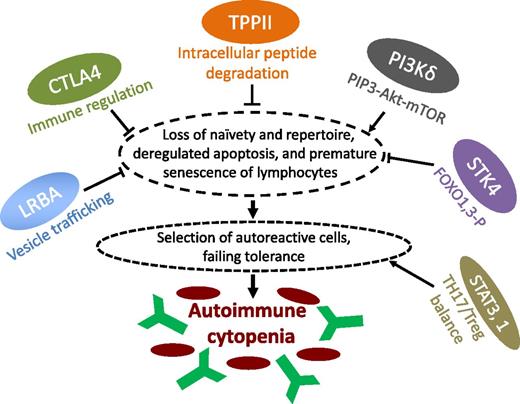
Newly identified and potentially targetable mechanisms of immune cytopenias in PIDs. In this issue of Blood, Stepensky et al characterize the immune phenotype underlying a novel PID with early-onset Evans syndrome due to loss of a central cytoplasmic peptidase, TPPII.1 Stepensky et al show that a stress-induced immunosenescence program is induced in T and B lymphocytes of human subjects with TPPII deficiency. This is also observed in other cell types, including fibroblasts, as well as in TPPII-deficient mice.1 Together with a reduction of the antigen receptor diversity, loss of naive lymphocytes, reduced lymphocyte proliferative capacity, and deregulated apoptosis, this leads to an accumulation of autoreactive cells causing autoimmunity that largely resembles other newly described PIDs with cytopenia, such as LRBA deficiency, CTLA4 deficiency, PI(3)K hyperactivity, and loss of STK4,4-7 as indicated. Of note, an immunosenescence-like abnormal differentiation of T cells has also been identified recently in one of the examples par excellence of autoimmune cytopenias, namely, ALPS-Fas.8 Many of these newly defined pathomechanisms of autoimmunity in PIDs will enable the use of existing drugs or the development of new targeted drugs. This may include complementation of CTLA4 deficiency for immune regulation, interfering with the Akt/mTOR pathway, or pharmacologically influencing the transcriptional programs and cytokine networks (eg, interleukin-6) behind the TH17-Treg balance (as in the case of lost peripheral regulatory T cells due to STAT3 gain of function).9
Newly identified and potentially targetable mechanisms of immune cytopenias in PIDs. In this issue of Blood, Stepensky et al characterize the immune phenotype underlying a novel PID with early-onset Evans syndrome due to loss of a central cytoplasmic peptidase, TPPII.1 Stepensky et al show that a stress-induced immunosenescence program is induced in T and B lymphocytes of human subjects with TPPII deficiency. This is also observed in other cell types, including fibroblasts, as well as in TPPII-deficient mice.1 Together with a reduction of the antigen receptor diversity, loss of naive lymphocytes, reduced lymphocyte proliferative capacity, and deregulated apoptosis, this leads to an accumulation of autoreactive cells causing autoimmunity that largely resembles other newly described PIDs with cytopenia, such as LRBA deficiency, CTLA4 deficiency, PI(3)K hyperactivity, and loss of STK4,4-7 as indicated. Of note, an immunosenescence-like abnormal differentiation of T cells has also been identified recently in one of the examples par excellence of autoimmune cytopenias, namely, ALPS-Fas.8 Many of these newly defined pathomechanisms of autoimmunity in PIDs will enable the use of existing drugs or the development of new targeted drugs. This may include complementation of CTLA4 deficiency for immune regulation, interfering with the Akt/mTOR pathway, or pharmacologically influencing the transcriptional programs and cytokine networks (eg, interleukin-6) behind the TH17-Treg balance (as in the case of lost peripheral regulatory T cells due to STAT3 gain of function).9
Immune dysregulation and autoimmunity are being recognized as features of a growing number of primary immunodeficiency (PID) diseases that often add to an impaired capacity to fend off infections. Symptoms arising from autoimmunity, however, may also present as the first or only sign of PID. It is counterintuitive why an aging immune system fails in both self-defense and self-recognition: it is less capable of fighting infections, responding to vaccines, or eliminating tumor cells but more prone to the development of autoimmune conditions, such as rheumatoid arthritis (reviewed in Goronzy et al2 ). Altered homeostasis results in age-associated changes within the lymphocyte repertoire; these are detectable and occur prematurely in states of autoimmunity.2 The cellular changes in rheumatologic autoimmune diseases appear to be associated with telomere erosion and thus with replicative senescence.2 This is in contrast to the stress-induced or “acute” senescence which is proposed to occur in TPPII deficiency. However, shared features include an increased proportion of terminally differentiated effector memory T cells, loss of naivety, a contracted antigen receptor repertoire, deregulated apoptosis, and impaired lymphocyte proliferation.1 Physiologic induction of cell-cycle arrest, senescence, or apoptosis is an evolutionarily conserved cell fate decision. This is executed in response to various stresses in a manner dependent on the tissue type as well as the kind and degree of stress. In the pathological context of TPPII deficiency, the lymphocyte repertoire is skewed toward terminally differentiated and senescent phenotypes. This may result from both an impaired stress response per se and abrogated processing of peptides, which are not (further) degraded by the proteasome, such as cytotoxic T lymphocyte antigens and epitope precursors required for presentation by major histocompatibility complex class I.3 The surviving “end-stage” lymphocytes not only are inefficient to combat infections but also appear to have been selected for autoreactive clones! It remains to be elucidated whether cellular senescence in the context of TPPII deficiency is a direct and cell-intrinsic event and how its molecular mechanism relates to similar immunosenescence phenotypes observed in recently discovered primary immunodeficiencies associated with immune cytopenia, eg, as deficiency of the vesicle-trafficking– and autophagy-linked lipopolysaccharide-responsive beige and anchor-containing (LRBA) protein, haploinsufficiency of cytotoxic T-lymphocyte antigen 4 (CTLA4), gain of function of phosphatidylinositol-3-OH kinase (PI(3)K) p110delta or loss of PI(3)K p85alpha, or deficiency of serine threonine kinase 4 (STK4; see figure).4-7 Recently, a senescence-like disturbance of T-cell development was also reported in the paramount example of autoimmune cytopenia in PID, ie, autoimmune lymphoproliferative syndrome (ALPS) due to Fas deficiency.8 It is not clear if immunosenescence serves as a sufficient cause of autoimmune cytopenia. An array of mechanisms which are not linked to immunosenescence may contribute to the cytopenia in PID. These include a tipped balance between TH17 and regulatory T cells, most recently reported in gain-of-function mutations of signal transducer and activator of transcription 3,9 several syndromes of immune dysregulation and hemophagocytosis, syndromes of bone marrow failure, and secondary myelosuppression (reviewed in Seidel10 ). Thus, detection of markers of immunosenescence should be considered a valuable tool that, in addition to the determination of naive T cells, will support the immune phenotypic differential diagnosis. Although not routine, this should be feasible in many laboratories. When done in suspected PID, it should be of particular interest in the context of autoimmune cytopenias of childhood. A pertinent catalog of diseases will also pave the way to clarifying the signaling pathways that underlie immunosenescence and autoimmune cytopenia. The figure provides a sketch of candidate pathways that may be targeted by small compounds or antibodies. It is safe to assume that eventually, patients with chronic immune cytopenias will be the beneficiaries of these investigations.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal