In this issue of Blood, Goettel and colleagues introduce a novel humanized mouse model of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, whereby mice lacking murine major histocompatibility complex class II (MHC II) and expressing human HLA-DR1 (NOD.PrkdcscidIl2rγ−/−H2-Ab1−/−; NSGAb°DR1) are reconstituted with hematopoietic stem cells (HSCs) from a patient with IPEX syndrome to generate a humanized model for primary immune deficiency presenting as fatal autoimmunity.1
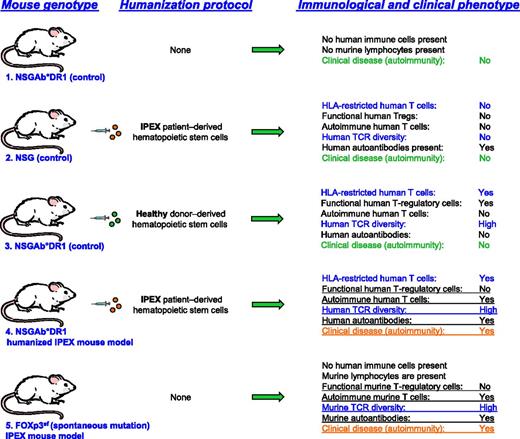
Overview of the generation of the HLA-DR1-NSG humanized IPEX syndrome mouse models, appropriate controls, and disease phenotypes. TCR, T-cell receptor.
Overview of the generation of the HLA-DR1-NSG humanized IPEX syndrome mouse models, appropriate controls, and disease phenotypes. TCR, T-cell receptor.
“Humanized mice” allow the study of human immune cells in the context of human diseases for the evaluation of organ-specific pathologies for which human samples may not be available or accessible, and for the assessment of experimental approaches that may be harmful when performed in patients or human volunteers. Thus, humanized mice have become a valuable translational research tool for the study of pathogen transmission, immunopathogenesis, viral latency, pathogen evolution, immune escape, prevention therapy assessments, and vaccine development.2 Humanization of tissues in mice is usually achieved by the transplant of human tissues and/or HSCs into lymphopenic hosts. The resulting cohort of humanized mice is genetically identical (individual members within a mouse strain are genetically identical due to a century of sibling inbreeding),3 and several dozen humanized mice can be generated from 1 human donor. Now, adoptive transfers of isolated human lymphocytes can be performed without rejection by the recipient immune system, and antibody-mediated in vivo lymphocyte depletion enables the assessment of the importance of individual immune cell types or pathways.
Several sophisticated humanized mouse models that differ in host genetics and/or their reconstitution methods have been established, including (1) mice that allow the evaluation of functional human innate and adaptive immune responses to infection and/or vaccination2 ; (2) mice that enable the humanization of hepatocytes and the study of hepatotropic pathogens4 such as malaria parasites or hepatitis viruses; and (3) germfree mice, in which a human microbiome has been established by oral fecal transplant.5 However, translational autoimmune disease models are rare, with the exception of graft-versus-host disease models, and currently, no humanized mouse models exist to study primary immune deficiency disorders, which present as autoimmune diseases.
In a particularly elegant study in the current issue of Blood, Goettel and colleagues describe the development and disease phenotype of a novel humanized mouse model of a primary immunodeficiency called IPEX syndrome.1 IPEX syndrome is a heritable X-linked recessive disorder caused by a number of mutations, mostly missense, found in the DNA-binding domain of the forkhead box P3 (FOXP3) protein.6 The FOXP3 protein is required for the development and function of CD4+CD25+ regulatory T cells (Tregs) derived from both the thymus Tregs and periphery Tregs. FOXP3-expressing Tregs are critical in the establishment and maintenance of peripheral self-tolerance. Therefore, the immunopathology of patients with IPEX syndrome is swift, often presenting between 1 and 3 months of age, and includes autoimmune enteropathy; endocrinopathy; eczematous dermatitis, which manifests as excessive cytokine production; elevated immunoglobulin (Ig)E levels; and chronic inflammation, leading to death. The various mutations within FOXP3 can lead to multiple functional fates, including a loss of transcriptional repression, messenger RNA stability, DNA binding, and FOXP3 dimerization. Goettel et al have significantly advanced the study of IPEX syndrome in vivo and, potentially, any hematopoiesis-derived disease, in their newly generated NSGAb°DR1 mouse model.
Mice that carry the scurfy mutation have defective FOXP3 that closely resembles IPEX syndrome, including widespread autoimmunity and high quantities of lymphocyte infiltrates within the lung, pancreas, stomach, skin, liver, and gut.7 However, the recapitulation of IPEX within mice using HSCs from IPEX patients has not been reported. In this issue, Goettel and colleagues successfully transplanted CD34+ cells into an NOD.PrkdcscidIl2rγ−/− (NSG) host that expresses the human MHC II HLA-DR1 (NSGAb°DR1), in the absence of murine MHC II (HLA-DR1-NSG; see figure). The lack of murine MHC II presumably allows for the full maturation of T and B cells and promotes T-cell help to B cells and full immune function. Indeed, Goettel and colleagues demonstrate that when CD34+ cells are engrafted into the HLA-DR1–expressing NSG mice, there is a significant improvement in both mature B- and T-lymphocyte development and function. Importantly, the HLA-DR1–expressing mice are able to develop delayed-type hypersensitivity, exhibiting increased IgG and IgE levels. To test whether this improved adaptive immune response could recapitulate a spontaneous and defined immunologic disorder, Goettel and colleagues transplanted HSCs from an IPEX patient. Remarkably, nearly all of the IPEX(DR1) (NSGAb°DR1) mice succumbed to multiorgan inflammation due to autoantibody production and the lack of a functional Treg compartment (see figure). It is important to note that IPEX(NSG) mice without the HLA-DR1–expressing allele displayed an increase in serum antibodies and an overall absolute increase in T-cell numbers; however, these mice did not develop widespread autoimmunity. This result is particularly significant because it highlights the importance of an intact mature lymphocyte compartment in the establishment of a fully adaptive immune response.
There are many diseases that will benefit from the advancement of immune disease–specific humanized mice, including monogenic immune disorders in which there is reliance on the adaptive immune arm with an intact mature T- and B-cell compartment. The newly developed NSGAb°DR1 mouse emphasizes just how invaluable a mouse model can be to dissect complex pathological disorders, including IPEX syndrome and other rare monogenic immune disorders, diabetes, and inflammatory bowel disease.8 By utilizing CD34+ HSCs, Goettel and colleagues are able to select T cells in the thymus on HLA-DR1, although the donor population was HLA mismatched. The ability to study the development of a spontaneous disease in-depth, before onset and during the development of pathogenesis, will move science forward immensely. In particular, the NSGAb°DR1 mouse will provide novel information about the initiation of IPEX due to particular point mutations in FOXP3, including direct and indirect involvement of Tregs in B-cell maturation and autoantibody production, and interactions with other immune populations including natural killer cells and macrophages. It will be interesting to learn in future studies how closely NSGAb°DR1-derived IPEX Tregs functionally behave in comparison with human-derived IPEX Tregs. Can NSGAb°DR1 Tregs express similar amounts of inhibitory cytokines (interleukin [IL]-35, IL-10, and transforming growth factor β) and inhibitory molecules (programmed death ligand 1 and cytotoxic T-lymphocyte–associated protein 4)?9,10 Thus, NSGAb°DR1 mice, alone or in combination with additional HLA molecules and/or other human cytokines, will enable in-depth analyses of developmental and functional defects that underlie immunodeficiencies and autoimmune disorders, and much-needed evaluations of emerging immunotherapies in a preclinical translational animal model.
Conflict-of-interest disclosure: The authors declare no competing financial interests.