Key Points
SCID-X1 patients treated with gene therapy show faster T-cell development compared with patients treated with haploidentical HSCT.
Abstract
During the last decade, gene therapy via ex vivo gene transfer into autologous hematopoietic stem cells has emerged as a convincing therapy for severe combined immunodeficiency caused by ILR2G mutation (SCID-X1) despite the occurrence of genotoxicity caused by the integration of first-generation retroviral vectors. However, the place of gene therapy among the therapeutic armamentarium remains to be defined. We retrospectively analyze and compare clinical outcomes and immune reconstitution in 13 consecutive SCID-X1 patients having undergone haploidentical hematopoietic stem cell transplantation (HSCT) and 14 SCID-X1 patients treated with gene therapy over the same period at a single center level: the Necker Children’s Hospital (Paris, France). Our results show a clear advantage in terms of T-cell development of gene therapy over HSCT with a mismatched donor. Patients treated with gene therapy display a faster T-cell reconstitution and a better long-term thymic output. Interestingly, this advantage of gene therapy vs haploidentical HSCT seems to be independent of the existence of clinical graft-versus-host disease in the latter condition. If data of safety are confirmed over the long term, gene therapy for SCID-X1 appears to be an equal, if not superior, alternative to haploidentical HSCT.
Introduction
X–linked severe combined immunodeficiency (SCID-X1) is the most frequent genetic form of SCID and accounts for 30% to 40% of cases.1 SCID-X1 is caused by defects in IL2RG, the gene encoding the interleukin (IL-2) receptor γ chain (γc). The common γc deficiency is typically characterized by a complete block in the development of T cells and natural killer (NK) lymphocytes and thus the absence of these cell lineages.2 If untreated, SCID-X1 leads to death within the first year of life. Nonconditioned, allogeneic, hematopoietic stem cell transplantation (HSCT) has been the gold standard therapy for this disorder since 1968. However, the outcome of HSCT is highly dependent on the availability of a suitable hematopoietic stem cell (HSC) donor: the 3-year overall survival rate is 90% to 97% when the donor is a geno-identical sibling but only 66% to 79% with an alternative donor.1,3,4 The presence of an active infection at time of treatment is also strongly associated with a lower survival rate after HSCT: 50% of 5-year survival after HSCT vs 80%.4
Gene therapy (GT; via the transduction of a corrected copy of a gene into autologous HSCs) has been considered as an attractive approach to overcome the absence of a suitable donor. The efficacy of γc gene transfer in autologous HSCs from SCID-X1 patients has been clearly demonstrated by the results of the early trials. Seventeen of the 20 treated subjects are alive and display full (or nearly full) correction of their T-cell immunodeficiency with a median follow-up of 12 years (7-15.5 years).5,6 However, these studies were hampered by the occurrence of genotoxicity resulting from oncogene transactivation by the viral long terminal repeat (LTR).7 Therefore, a new strategy has been developed based on a self-inactivating (SIN) γ-retroviral construct.7-11 The procedures of haplo-HSCT and GT have not yet been compared. Hence, we decided to retrospectively analyze and compare clinical outcomes and immune reconstitution in (1) 13 SCID-X1 patients having undergone HLA-mismatched HSCT between January 2000 and December 2013; and (2) 14 SCID-X1 patients having undergone GT over the same period of time. All patients had been treated at Necker Children’s Hospital (Paris, France). Our results show a clear advantage of GT over HSCT with a mismatched donor in terms of the time course and perhaps of quality of immune reconstitution.
Methods
We included 27 consecutive patients diagnosed with γc deficiency between March 1999 and December 2013. All patients lacked an HLA identical donor. Informed consent for participation in the study was obtained from the patient’s parent(s), in accordance with French regulatory requirements and the Declaration of Helsinki. All patients were placed in sterile isolation and received prophylactic treatment with cotrimoxazol and intravenous immunoglobulins.
Fourteen of the patients were participating in 2 prospective trials of GT that had been approved by the French drug agency and the local independent ethics committee. Autologous genetically modified CD34+ bone marrow cells were prepared as described elsewhere.5,11
In the first trial, which took place from March 1999 to April 2002, patients (GT1-GT9) received ex vivo-transduced CD34 cells in the absence of any additional treatment. The vector used was a first generation γ-retrovirus in which the common γc cytokine receptor subunit had been placed under the transcriptional control of the viral LTR. Patients with SCID-X1 who lacked an HLA identical donor were all eligible for enrollment in the study. The trial’s results and outcomes have been previously reported.5,6,12,13 Another group of patients (GT10-GT14) was treated in a second trial based on a SIN γ-retrovirus, which started in November 2010 and ended in June 2012.11 Only patients without an HLA-matched donor and presenting with an active infection were eligible for enrollment. Active infections were defined as protracted viral lung infection, cytomegalovirus infection, adenovirus and Epstein-Barr virus infection, and disseminated Bacillus Calmette-Guérin (BCG) infection. These patients also received ex vivo-transduced CD34 cells in the absence of any additional treatment except for patient GT11. This patient received 2 doses of fludarabine (total, 80 mg/m2 of body surface area) on days −3 and −2 before receiving transduced cells to reduce the massive number of maternally engrafted T cells. From March 1999 to December 2013, 13 other γc-deficient patients lacking a matched sibling donor were treated by HSCT with a haploidentical donor according to the European Bone Marrow Transplantation/European Society of Immune Disorder guidelines for primary immunodeficiencies. For these patients, haploidentical HSCT was undertaken because of the unavailability of GT (between 2002 and 2010) or because the patient did not meet the inclusion criteria of severe ongoing infection (between 2010 and 2013). These 13 patients received 2 infusions of 2.5 mg/kg rabbit antithymoglobulin (r-ATG, Thymoglobuline; Genzyme) on days −2 and −1 as conditioning. Donor and recipient HLA status was determined by low-resolution class I and high-resolution class II molecular DNA typing. T-lymphocyte depletion of the harvested bone marrow was performed by CD34 immunomagnetic selection with a Clinicmacs system (Miltenyi Biotec, Bergisch Gladbach, Germany). Only patients with T-cell reconstitution were included in the statistical analysis. Thus, 2 patients were excluded from this analysis in the GT group (GT3 and GT13) and 3 patients in the haploidentical HSCT group (P1, P11, and P13). Acute graft-versus-host disease (GVHD) was assessed and graded according to established standardized criteria.14
Results
Characteristics of the population
Twenty-seven patients with SCID-X1 were included in the study. The median age at the time of GT or haploidentical HSCT was 7 months (range, 1-15 months), with no statistically significant difference between the 2 groups (Table 1). Six patients in the GT group and 4 in the haploidentical HSCT group had detectable maternal T cells (supplemental Tables 1 and 2, available on the Blood Web site). There was no significant intergroup difference in the median dose of CD34+ γc-positive cells received (7 × 106/kg in the haploidentical HSCT group and 8.9 × 106/kg in the GT group). Patients treated with GT presented with slightly more frequent active infections (9 of 14) than patients treated with haploidentical HSCT (5 of 13). Two patients in the GT group had been treated with rituximab for Epstein-Barr virus-induced lymphoproliferative disease before GT.
Patient’s characteristics
| . | Gene therapy (n = 14) . | Haploidentical HSCT (n = 13) . |
|---|---|---|
| Median age in months (range) | 8 (1-11) | 7 (1-15) |
| Active infections at treatment | 9 | 5 |
| Maternal T cells (/µL): median (range) | 0 (0-8051) | 0 (0-270) |
| CD34+ cell dose (×106/kg): median (range) | 8.9 (1-22) | 7 (2.4-17) |
| Adverse events | ||
| Graft failure | 1 | 3 |
| GVHD | — | 4 |
| Dysimmunity | — | 3 |
| T-ALL | 4 | — |
| Time course of BCG infection in months: median (range) | 11 (8.9-14.6) | 25.5 (24.1-28.7) |
| Infection-related hospitalization duration: day per patient per year | 0.03 | 0.4 |
| Death | 2 | 2 |
| Median duration of follow-up in years (range) | 12 (1-15) | 6 (1-12) |
| . | Gene therapy (n = 14) . | Haploidentical HSCT (n = 13) . |
|---|---|---|
| Median age in months (range) | 8 (1-11) | 7 (1-15) |
| Active infections at treatment | 9 | 5 |
| Maternal T cells (/µL): median (range) | 0 (0-8051) | 0 (0-270) |
| CD34+ cell dose (×106/kg): median (range) | 8.9 (1-22) | 7 (2.4-17) |
| Adverse events | ||
| Graft failure | 1 | 3 |
| GVHD | — | 4 |
| Dysimmunity | — | 3 |
| T-ALL | 4 | — |
| Time course of BCG infection in months: median (range) | 11 (8.9-14.6) | 25.5 (24.1-28.7) |
| Infection-related hospitalization duration: day per patient per year | 0.03 | 0.4 |
| Death | 2 | 2 |
| Median duration of follow-up in years (range) | 12 (1-15) | 6 (1-12) |
Clinical outcomes
The median follow-up period was 12 years (range, 1-15 years) for GT patients and 6 years (range, 1-12 years) for haploidentical HSCT patients (P = .12). Graft failure was observed in 3 haploidentical HSCT patients. These patients were transplanted a second time with their other parent as donor. Two of the 3 patients received a full myeloablative conditioning regimen 6 and 12 months after the first HSCT. There was no significant difference in circulating T- or NK-cell counts before HSCT between these 3 cases and the other 10 patients. One graft failure was observed in the GT group. The patient (GT3) presented with persistent splenomegaly caused by a disseminated bacillus Calmette-Guerin infection. He failed to reconstitute his T-cell compartment likely because of trapping of progenitor cells in the spleen. After splenectomy, he successfully underwent HSCT with an unrelated donor 8 months after GT.
Resolution of disseminated BCG infection was fastest in GT patients (median, 11 months; range, 8.9-14.6 months) compared with haploidentical HSCT patients (median, 25.5 months; range, 24.1-28.7 months; P = .029). After the treatment, infection-related hospitalization occurred in 3 patients of the GT group (for a total of 3 admissions), whereas it occurred in 5 patients of the haploidentical transplanted group (for a total of 15 admissions). Infections leading to hospitalization were BCG infection (n = 2) and bacterial pneumonia (n = 1) in the GT group. In the haploidentical HSCT group, patients were admitted for isolated fever (n = 3), viral gastroenteritis (n = 2), sepsis (n = 4), bacterial pneumonia (n = 1), and BCG infection (n = 5). The number of days of infection-related hospitalization was 0.4 and 0.03 days per patient per year in the haploidentical HSCT and GT groups, respectively (P = .001).
Seven of the 13 haploidentical HSCT patients developed treatment-related complications. Four presented with acute grade 2 GVHD. Immune dysfunction occurred in 3 patients (immune hemolytic anemia, allo-immune hepatitis, and dysimmune enteropathy) and was associated with death in 1 patient (P6) (Table 1; supplemental Table 3).
Two patients treated with haploidentical HSCT died: P1, 6 months after transplantation from respiratory viral infection; and P6, 7 years after transplantation from severe dysimmune enteropathy caused by poor immune reconstitution. Two patients in the GT group died. GT13 died of adenoviral infection at 4 months after therapy, and GT4 died as a result of chemoresistant T-cell acute lymphoblastic leukemia 5 years after GT.5,8
T-cell development
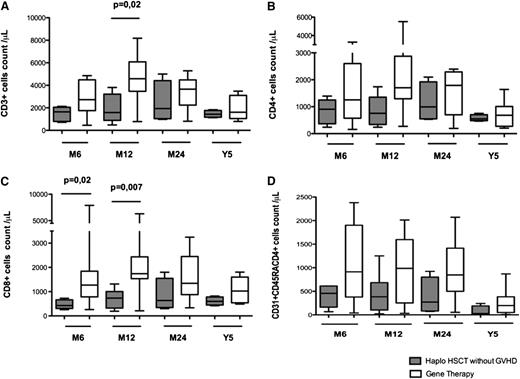
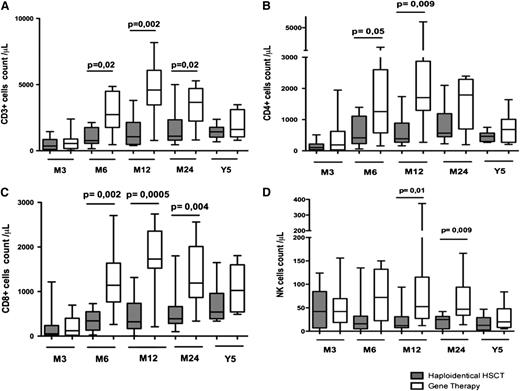
T-cell reconstitution was faster in GT patients compared with haploidentical HSCT patients (Figure 1). Six months after transplantation, T-cell counts had reached normal values for age in 11 of the 14 GT patients (78%) and 4 of the 15 haploidentical HSCT patients (26%). Counts of CD3, CD4, and CD8 cells were significantly higher 6 and 12 months after GT than after haploidentical HSCT (Figure 1A-C). These differences had disappeared 5 years after transplantation, with normal numbers of circulating T cells in 7 GT patients and 9 haploidentical HSCT patients (Figure 1). The thymic output of CD4+ T lymphocytes (estimated by the absolute count of CD4+ lymphocytes expressing the CD31 and CD45RA markers) was also significantly higher in GT patients than haploidentical HSCT patients 6 and 12 months after transplantation (Figure 2A). This difference remained significant at 5 years (median CD31+CD45RA+ cells in 7 GT patients, 198/μL [range, 8-383] vs 16/μL [range, 3-869] in 9 haploidentical HSCT patients; P = .03; Figure 2A). Similarly, the absolute count of naïve CCR7+CD45RA+CD8+ lymphocytes was higher in GT patients (229/μL; range, 57-520) than in haplotransplanted patients (33/μL; range, 1-118) 5 years after transplantation (P = .008).
T- and NK-cell development after haploidentical HSCT or GT. This figure shows the absolute peripheral blood cell count in patients at 3, 6, 12, and 24 months and 5 years after GT or haploidentical HSCT with regard to (A) CD3+ lymphocytes, (B) CD3+CD4+ lymphocytes, (C) CD3+CD8+ lymphocytes, and (D) CD3−CD16+CD56+ lymphocytes. Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
T- and NK-cell development after haploidentical HSCT or GT. This figure shows the absolute peripheral blood cell count in patients at 3, 6, 12, and 24 months and 5 years after GT or haploidentical HSCT with regard to (A) CD3+ lymphocytes, (B) CD3+CD4+ lymphocytes, (C) CD3+CD8+ lymphocytes, and (D) CD3−CD16+CD56+ lymphocytes. Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
Thymic output and T-cell functionality after haploidentical HSCT or GT. (A) Absolute number of peripheral blood CD3+CD4+ lymphocytes expressing CD45RA and CD31 in haploidentical HSCT patients and GT patients 6, 12, and 24 months and 5 years after GT or haplotransplantation. (B) Absolute number of peripheral blood CD3+CD8+ lymphocytes expressing CCR7 and CD45RA at 5 years after treatment. (C) Lymphocyte proliferation in response to phytohemagglutinin 12 months after procedure (left) and tetanus toxoid after immunization (right) in 4 haploidentical HSCT patients and 9 GT patients. (D) Absolute number of CD4+CD25+CD127lowFoxp3+CD4+ T lymphocytes in haploidentical HSCT patients and GT patients at last follow-up (for GT patients: median of 3.5 years [range, 1.5-15 years], for haplotransplanted patients: median of 6.5 years [range, 4.5-8 years]). Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
Thymic output and T-cell functionality after haploidentical HSCT or GT. (A) Absolute number of peripheral blood CD3+CD4+ lymphocytes expressing CD45RA and CD31 in haploidentical HSCT patients and GT patients 6, 12, and 24 months and 5 years after GT or haplotransplantation. (B) Absolute number of peripheral blood CD3+CD8+ lymphocytes expressing CCR7 and CD45RA at 5 years after treatment. (C) Lymphocyte proliferation in response to phytohemagglutinin 12 months after procedure (left) and tetanus toxoid after immunization (right) in 4 haploidentical HSCT patients and 9 GT patients. (D) Absolute number of CD4+CD25+CD127lowFoxp3+CD4+ T lymphocytes in haploidentical HSCT patients and GT patients at last follow-up (for GT patients: median of 3.5 years [range, 1.5-15 years], for haplotransplanted patients: median of 6.5 years [range, 4.5-8 years]). Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
One year after transplantation, lymphocyte proliferation in response to phytohemagglutinin had achieved normal levels (ie, >50 × 103 cpm) in all 11 evaluated GT patients but in only 4 of the 10 evaluable haploidentical HSCT patients (Figure 2C). However, there was no difference in lymphocyte proliferation in response to tetanus toxoid between patients treated with GT or haploidentical HSCT after immunization (Figure 2C).
The median count of circulating regulatory CD4+ lymphocytes (defined as CD4+CD25+CD127lowFoxp3+ lymphocytes) was significantly higher in GT patients than in haploidentical HSCT patients (62/μL and 8.5/μL, respectively; P = .01) at last follow-up (median of 3.5 years [range, 1.5-15] and 6.5 years [range, 4.5-8], respectively; Figure 2D).
Comparison of immune reconstitution between haplotransplanted patients with (n = 4) or without clinical GVHd (n = 9) showed a slower T-cell reconstitution in patients with GVHD with lower thymic output. Mainly because of the small size of cohorts, these differences were not statistically significant (supplemental Figure 1). After excluding patients with clinical signs of GVHD, we still observed a trend of faster T-cell compartment development in patients treated with GT compared with haplotransplanted patients (Figure 3).
Comparison of T-cell development in haplotransplanted patients without GVHD or GT-treated patients. The absolute peripheral blood (A) CD3+, (B) CD3+CD4+, and (C) CD3+CD8+ lymphocyte count in haplotransplanted patients without clinical GVHD and GT patients at 6, 12, and 24 months and 5 years. (D) Absolute number of peripheral blood CD3+CD4+ lymphocytes expressing CD45RA and CD31 in haploidentical HSCT patients without GVHD or GT patients at 6, 12, and 24 months and 5 years after transplantation. Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
Comparison of T-cell development in haplotransplanted patients without GVHD or GT-treated patients. The absolute peripheral blood (A) CD3+, (B) CD3+CD4+, and (C) CD3+CD8+ lymphocyte count in haplotransplanted patients without clinical GVHD and GT patients at 6, 12, and 24 months and 5 years. (D) Absolute number of peripheral blood CD3+CD4+ lymphocytes expressing CD45RA and CD31 in haploidentical HSCT patients without GVHD or GT patients at 6, 12, and 24 months and 5 years after transplantation. Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
Development of the NK-cell compartment
Reconstitution of the NK-cell compartment did not occur in any of the patients in either group, with NK-cell counts well below the normal-for-age values. However, GT patients displayed higher NK-cell counts than haploidentical HSCT patients. This higher count was significant at 12 and 24 months (P = .01 and 0.009, respectively) after therapy (Figure 1D). This difference was no longer observed 5 years after treatment (Figure 1D). The NK cells phenotypical pattern was very homogeneous in each group. The CD3–CD56dimCD16bright population was almost absent in GT and haplotransplanted patients, whereas it represents the dominant NK subpopulation in healthy subjects. Furthermore, expression of CD56 by NK cells was reduced in haplotransplanted patients compared with GT patients and healthy controls. Expression of NKp46 and NKG2D was also diminished in CD3−CD56+ cells from haplotransplanted and GT patients compared with healthy controls (supplemental Figures 2 and 3).
Evaluation of the B-cell compartment
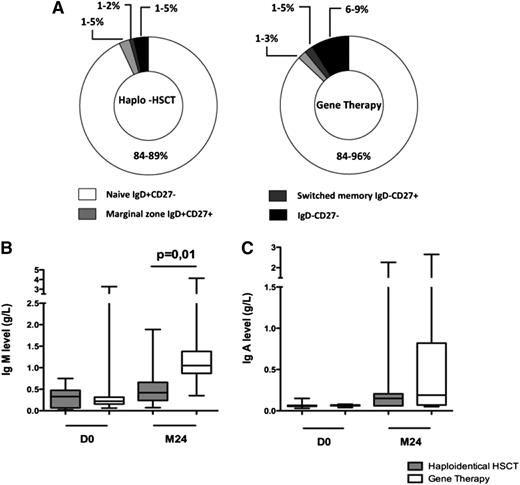
As expected (given the absence of a conditioning regimen required for effective HSC engraftment), the B-cell function remained defective in all patients. Repartition of CD19+ cell subpopulation (ie, naive immunoglobulin [Ig]D+CD27−, marginal zone IgD+CD27+, switched memory IgD−CD27+, and IgD−CD27− cells) was not statistically different between patients treated with GT and haploidentical HSCT (Figure 4A). Twenty-four months after transplantation, levels of IgM were higher in GT patients (median, 1.05 g/L; range, 0.35-4.3 g/L) than in haploidentical HSCT patients (median, 0.42; range, 0.06-1.89 g/L; P = .0151; Figure 4B). There was no significant intergroup difference in IgA levels (Figure 4C). Comparison of IgG levels was not relevant, because most patients were still receiving intravenous immunoglobulin (IVIG) replacement therapy. However, among the patients evaluable for long-term B-cell reconstitution, IVIG replacement therapy was not withdrawn in any of the 10 haploidentical HSCT patients but was withdrawn in 4 of the 12 GT patients based on clinical evidence (all of whom had been treated with the LTR-driven γ-retrovirus).
B-cell immune function. (A) CD19+ cell subpopulations in patients treated with haploidentical HSCT and GT. Percentage range of each subpopulation (ie, naives IgD+CD27−, marginal zone IgD+CD27+, switched memory IgD−CD27+, and IgD−CD27− cells) is indicated. (B) IgM levels (g/L) on day 0 and at 2 years in haploidentical HSCT patients and GT patients. (C) IgA levels (g/L) on day 0 and at 2 years in haplo-identical HSCT patients and GT patients. Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
B-cell immune function. (A) CD19+ cell subpopulations in patients treated with haploidentical HSCT and GT. Percentage range of each subpopulation (ie, naives IgD+CD27−, marginal zone IgD+CD27+, switched memory IgD−CD27+, and IgD−CD27− cells) is indicated. (B) IgM levels (g/L) on day 0 and at 2 years in haploidentical HSCT patients and GT patients. (C) IgA levels (g/L) on day 0 and at 2 years in haplo-identical HSCT patients and GT patients. Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.
Discussion
We compared clinical and immunological outcomes in 14 SCID-X1 patients having undergone with retrovirus-mediated GT and 13 SCID-X1 patients having undergone haploidentical HSCT in the same center between January 2000 and December 2013. Haploidentical donors are the main source of HSCs for transplantation in SCIDs when SCID patients lack a geno-identical sibling; indeed, haploidentical donors accounted for 96 of the 181 transplants recorded in the Stem CEll Tranplantation for Primary Immune Deficiency Registry between 2000 and 2005 and 138 of the 240 transplants of the US registry between 2000 and 2009.1,4,16 This preponderance is due to the easier availability of haploidentical donors (relative to other unrelated sources) and the superimposable outcomes of HSCT with haploidentical or mismatched unrelated donor.1,4 GT has emerged as an attractive alternative to circumvent the risk of immune conflict in the setting of allogeneic transplantation.
Although the efficacy of γc gene transfer in autologous HSC of SCID-X1 patients has been previously reported, the rank of this procedure in the treatment hierarchy has yet to be defined.5,13 Our present study retrospectively compared outcomes in GT and haploidentical HSCT to establish whether GT is an appropriate alternative to haploidentical transplantation when the patient lacks a geno-identical sibling.
In these 2 populations of SCID-X1 patients with similar pretransplant profiles except for a slightly higher proportion of active infections in patients treated with GT (because of the inclusion’s criteria requirement), we found that GT provides faster immune reconstitution in the first year after transplantation than haploidentical HSCT. The faster immune reconstitution associated with GT is accompanied by a faster resolution of some opportunistic infections, such as disseminated BCG infection, and a lower rate of infection-related hospitalization compared with haploidentical HSCT. r-ATG is administered in case of haploidentical HSCT mainly to prevent the occurence of GVHD. As r-ATG is associated with a delayed immunological recovery related to lymphocyte depletion, one could wonder if it could account for immune reconstitution kinetics differences between the 2 procedures. However, we recently developed a pharmacokinetic-pharmacodymamic model of active rATG to assess the association between r-ATG concentrations and immune reconstitution (Naim Bouazza, unpublished data, 2015). According to this model, the administration of 2 consecutive doses of 2.5mg/kg of r-ATG ensures a maximum 6-day-long T-cell depletion. After this period, r-ATG remains at a sublympholytic level. Thus, it seems unlikely that r-ATG at the dose administered to the patients has a major influence on T-cell reconstitution. In addition, data on the kinetics of T-cell development in SCID patients who received haploidentical HSCT in the absence of ATG therapy do not differ from those herein reported.17-20
Furthermore, 5-year thymic output appeared to be more consistent in GT patients than in haploidentical HSCT patients. This difference in thymic output could be explained by an allogeneic reaction against the thymic tissue in patients treated with mismatched donors whether they present with clinical signs of GVHD or not.21 Furthermore, allogeneic transplantation is hampered by the risk of graft failure and immune dysfunction as well. In our series, 7 haploidentical HSCT patients presented immune complications that resulted in immunosuppressive therapy and further delayed immune reconstitution. Graft failure was observed in 3 haploidentical HSCT patients. One GT patient failed to reconstitute an adequate T-cell compartment because of trapping of the progenitor cells in the spleen. One major concern in GT is the genotoxicity observed in the early trials, because 4 patients in the first GT trial developed T-cell leukemia 2.5 to 5 years after the procedure. Occurrence of these adverse effects (related to vector integration) is likely to have been circumvented by recent advances in vector design.11 It is noteworthy that to date, none of the patients treated with a SIN γ-retrovirus have presented with genotoxicity. However, the long-term clinical application of these new vectors has yet to be characterized.
Even though GT appears to be superior to haploidentical HSCT in terms of T-cell reconstitution, reconstitution of both the B-cell and NK-cell compartments remained poor in both the GT and haploidentical HSCT groups. Although large studies have reported withdrawal of IVIG replacement therapy in two thirds of patients treated with haploidentical HSCT, all of our transplanted patients still require this therapy.22 Four patients treated during the first GT trial were able to quit IVIG replacement therapy, suggesting either the persistence of undetectable, transduced B cells or sufficient activation of γ-chain negative B cells by T-cell signals and γ-chain-independent cytokines (with the notable exception of IL-21).23-25 The absence of IVIG replacement withdrawal for patients treated in the second GT trial could also suggest that difference in strength of expression of the transgene by the 2 different vectors is an important factor in the correction of B-cell function. However, whether haploidentical HSCT or GT fails to permit the development of adequate B-cell function without donor B-cell chimerism needs to be further investigated.22,26 Thus, complete immune reconstitution appears to be unattainable in the absence of stem cell engraftment provided by myeloablative conditioning.22,27,28
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from INSERM and European Research Council.
Authorship
Contribution: F.T. designed the research, collected the data, participated in the clinical care of the patients, and wrote the manuscript; D.M., B.N., P.F., G.C., and S.B. participated in the clinical care of the patients and critically read the manuscript; R.C. and A.M. performed experiments, participated in the biological follow-up of patients treated with GT, collected the data, and critically read the manuscript; L.C., J.B., J.-M.L., and B.T. performed experiments, participated in the biological follow-up of patients treated with GT, and critically read the manuscript. C.P. performed genetic ad biologic diagnosis of the patient and critically read the manuscript; S.H.-B.-A. participated in design and writing of the manuscript; M.C. designed the research and critically read the manuscript; and A.F. participated in the clinical care of the patient and in writing the paper and critically read the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabien Touzot, Département de Biothérapie, Hôpital Necker-Enfants Malades, Paris 75015, France; e-mail: fabien.touzot@nck.aphp.fr; or Alain Fischer, Unité d’Immuno-Hématologie et Rhumatologie Pédiatrique Hôpital Necker-Enfants Malades, Paris 75015, France; e-mail: alain.fischer@nck.aphp.fr.
References
Author notes
D.M., R.C., and B.N. contributed equally to this work.
A.F., S.H.-B.-A., and M.C. contributed equally to this work.

![Figure 2. Thymic output and T-cell functionality after haploidentical HSCT or GT. (A) Absolute number of peripheral blood CD3+CD4+ lymphocytes expressing CD45RA and CD31 in haploidentical HSCT patients and GT patients 6, 12, and 24 months and 5 years after GT or haplotransplantation. (B) Absolute number of peripheral blood CD3+CD8+ lymphocytes expressing CCR7 and CD45RA at 5 years after treatment. (C) Lymphocyte proliferation in response to phytohemagglutinin 12 months after procedure (left) and tetanus toxoid after immunization (right) in 4 haploidentical HSCT patients and 9 GT patients. (D) Absolute number of CD4+CD25+CD127lowFoxp3+CD4+ T lymphocytes in haploidentical HSCT patients and GT patients at last follow-up (for GT patients: median of 3.5 years [range, 1.5-15 years], for haplotransplanted patients: median of 6.5 years [range, 4.5-8 years]). Extremities of the whiskers boxes represent minimum and maximum values. Bottom and top of the box represent the first and third quartiles, respectively. The horizontal bar in the box represents the median value.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/23/10.1182_blood-2014-12-616003/4/m_3563f2.jpeg?Expires=1767840232&Signature=dQuKqa17iH9hOYlCV09aMPLiVI273Vaytb4WXyzafyx4UAVYoMJRj8cegttRLroWaBt-J82RP~l0DxiU-uwiOoUvjgoCxcMknph1riMs3iD3o6QRSm4Lt9L0J5sYu4g82GeYo1~r2c9t~jmvF7QHCzqU1JB8Xuota7~nskd7WWcvr37LT86YuCnv4mp2jITkiWeDDjfwKWbpyFEikqL29yRDxjd~Pk37NK2u5fqL99K8V52uBAxu3yOkgy~JUz06rBgMn2D4Q~QYuD32uY9whDyBxAfrMJYJwUhT3PTZZdQA~upd1sfXF-zzy54i-JGJ5T-fghMaxrmpfCsEO2vtlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)