In this issue of Blood, Schneider et al1 document that sugar moieties, introduced into surface-membrane immunoglobulin (smIg) antigen-binding sites on follicular lymphoma (FL) cells by somatic hypermutation (SHM), induce smIg-mediated signals in a distinct, nonstandard manner. This signaling results from the sugar physically interacting with sugar-specific lectins from bacteria. The findings confirm and support the clinical relevance of previous observations about SHM introducing glycans into the variable domains of FL antigen-binding sites,2-5 and they support the potential importance of microbial exposure to lymphomagenesis and disease progression.
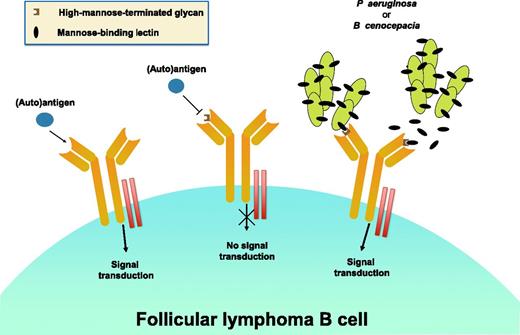
Three different antigen-binding possibilities on the surface membrane of a follicular lymphoma B cell and their anticipated functional consequences are shown. On the far left is the expected scenario. In the absence of an N-linked glycan, an (auto)antigen can interact with the antigen-binding site of the expressed smIg and, with the involvement of the CD79a/d complex ( ), transduce a downstream BCR-mediated signal. In the middle is the setting in which a high-mannose-terminated glycan in the antigen-binding site (
), transduce a downstream BCR-mediated signal. In the middle is the setting in which a high-mannose-terminated glycan in the antigen-binding site ( ) inhibits the binding of an (auto)antigen, due to either a conformational change in the antigen-binding site or steric hindrance based on the bulk of the glycan. In this instance, a BCR-mediated signal cannot be initiated. Finally, on the right is the situation in which either soluble or organism-bound mannose lectin (
) inhibits the binding of an (auto)antigen, due to either a conformational change in the antigen-binding site or steric hindrance based on the bulk of the glycan. In this instance, a BCR-mediated signal cannot be initiated. Finally, on the right is the situation in which either soluble or organism-bound mannose lectin ( ) interacts with high-mannose glycan in the antigen-binding site and induces a BCR signal. In the study by Schneider et al, these possibilities have been tested using monoclonal antibodies of defined specificity (before or after insertion of a high-mannose-terminated, N-linked glycan) or Igs derived from FL patients that contain in vivo SHM-induced high-mannose-terminated, N-linked glycans.
) interacts with high-mannose glycan in the antigen-binding site and induces a BCR signal. In the study by Schneider et al, these possibilities have been tested using monoclonal antibodies of defined specificity (before or after insertion of a high-mannose-terminated, N-linked glycan) or Igs derived from FL patients that contain in vivo SHM-induced high-mannose-terminated, N-linked glycans.
Three different antigen-binding possibilities on the surface membrane of a follicular lymphoma B cell and their anticipated functional consequences are shown. On the far left is the expected scenario. In the absence of an N-linked glycan, an (auto)antigen can interact with the antigen-binding site of the expressed smIg and, with the involvement of the CD79a/d complex ( ), transduce a downstream BCR-mediated signal. In the middle is the setting in which a high-mannose-terminated glycan in the antigen-binding site (
), transduce a downstream BCR-mediated signal. In the middle is the setting in which a high-mannose-terminated glycan in the antigen-binding site ( ) inhibits the binding of an (auto)antigen, due to either a conformational change in the antigen-binding site or steric hindrance based on the bulk of the glycan. In this instance, a BCR-mediated signal cannot be initiated. Finally, on the right is the situation in which either soluble or organism-bound mannose lectin (
) inhibits the binding of an (auto)antigen, due to either a conformational change in the antigen-binding site or steric hindrance based on the bulk of the glycan. In this instance, a BCR-mediated signal cannot be initiated. Finally, on the right is the situation in which either soluble or organism-bound mannose lectin ( ) interacts with high-mannose glycan in the antigen-binding site and induces a BCR signal. In the study by Schneider et al, these possibilities have been tested using monoclonal antibodies of defined specificity (before or after insertion of a high-mannose-terminated, N-linked glycan) or Igs derived from FL patients that contain in vivo SHM-induced high-mannose-terminated, N-linked glycans.
) interacts with high-mannose glycan in the antigen-binding site and induces a BCR signal. In the study by Schneider et al, these possibilities have been tested using monoclonal antibodies of defined specificity (before or after insertion of a high-mannose-terminated, N-linked glycan) or Igs derived from FL patients that contain in vivo SHM-induced high-mannose-terminated, N-linked glycans.
The retention of smIg on FL cells has been taken as an indication of the importance of ongoing signaling through B-cell receptor (BCR) immunoglobulins (Igs) for the maintenance and growth of such clones. Furthermore, FL B cells, as germinal center derivatives, can interact with other lymphoid cells such as T cells, myeloid-derived cells such as tumor-associated macrophages, follicular dendritic cells that display native (auto)antigen, and possibly interdigitating dendritic cells that present immunogenic peptides derived from (auto)antigen. An ongoing communication between FL cells and the tissue microenvironment is evidenced by the continued acquisition of immunoglobulin heavy-chain variable region (IGHV) gene mutations over time.6 Therefore, understanding the mechanisms whereby FL B cells interact with other cells in situ provides essential information about lymphoma cell biology and lays the groundwork for potential new prognostic and therapeutic approaches. The studies by Schneider et al accomplish both of these goals.
SHM can promote N-linked glycosylation by creating a new asparagine at a specific site or by changing the amino acids flanking an existing asparagine in the germline-encoded sequence. For each instance, the change leads to an “N-linked glycosylation sequon,” historically defined as Asn-Xaa-Ser/Thr (Xaa ≠ Pro), with the presence of Ser/Thr exerting the biggest influence on subsequent glycosylation.7 Changes that permit N-glycosylation can be found in ≥80% of FL clones2-5 ; these presumably occur before or at the time of lymphoma development, although new variants could appear subsequently based on ongoing SHM within the clone.
Using sophisticated molecular and cellular approaches that have been pioneered by the authors to understand the roles of BCR Ig signaling in the normal setting (eg, pre–B-cell biology8 ) or in other B-cell lymphoproliferative disorders (eg, chronic lymphocytic leukemia9 ), these new studies indicate that expression of sugar determinants on asparagine residues prevents binding by cognate antigens for BCR Igs of defined antigen specificity (see figure). This was demonstrated by manipulating the V-region DNA sequences of 3 murine Igs that react with either chemically defined haptens or proteins. The edited sequences contain N-linked glycosylation sequons that lead to expression of high-mannose sugars when inserted in a reporter lymphoid cell line. Control Igs, including those that were genetically manipulated but do not express glycans, did not inhibit cognate antigen binding. Furthermore, inhibition of cognate antigen binding, by steric or conformational effects due to the bulk of carbohydrate-modified asparagine, prevented antigen-induced BCR-mediated signaling via the manipulated test Igs.
Next, the authors demonstrated that smIgs from FL clones with mannose-terminating oligosaccharides could bind and be stimulated by exposure to mannose-specific lectins. This was done by expressing these Igs in the same reporter lymphoid cell line and subsequently exposing them to appropriate receptor molecules. Two soluble lectins with specificity for mannose derived from Pseudomonas aeruginosa and Burkholderia cenocepacia, organisms that are common in our everyday environment, were able to bind and signal through FL smIgs in the test system. Surprisingly, despite being able to bind to FL smIg-expressing cells, DC-SIGN, a mannose- binding lectin that is present on interdigitating dendritic cells, did not induce this type of signaling as previously reported in another experimental system.5 Thus, it remains unclear if FL cells can receive this type of BCR-signal induction and trophic input from autologous sugar-binding receptors.
Nevertheless, although the presence of N-linked glycans can inhibit (auto)antigen binding that might inhibit FL cell growth, the presence of these glycan residues permits interactions with carbohydrate binding structures on or from microbes, thereby generating signals in a carbohydrate class–specific manner that could lead to clonal expansion and possibly disease worsening (see figure). Thus, these studies directly support a potentially supportive role for this unique type of BCR signal induction (glycans with high-mannose interacting with mannose-binding molecules) in the biology of FL B cells. Furthermore, because normal B cells express N-linked glycans much less frequently,2 the development of FL might involve a selection for such modified smIgs.
Finally, the findings reported here1 and previously2-5 suggest approaches that might lead to better defined categorization of FL clones among patients and to potentially novel ways for prevention and therapeutic targeting. As suggested by the authors, “treatment with antibiotics to clear the opportunistic bacteria might represent a potential therapy for FL.”1 Therefore, identification of FL clones that exhibit/develop amino acid replacements leading to N-glycosylation sequons that might support “disadvantageous,” high-mannose sugar expression could allow a new type of patient subsetting. Patients bearing such clones might then be candidates for antibiotic prophylaxis. Alternatively, appropriate clones might be amenable to targeting the specific carbohydrate epitope by monoclonal antibodies or small molecules, should such reagents be made V-domain specific. Finally, it is intriguing to consider that the carbohydrate-specific IGHVDJ epitopes developed by SHM might represent neoantigens that could be recognized by autologous T lymphocytes. This recognition might support the outgrowth of a population of T cells that provide “help” for the survival and expansion of the FL clone; T cells with such specificities therefore might be therapeutic targets. Conversely, T-cell recognition could lead to cytotoxic cells recognizing the neoantigens; these cells might be harnessed therapeutically to control FL cell growth. T-cell recognition of IGHVDJ antigens, albeit presumably protein in nature, has been suggested as a means for clonal regulation in lymphoma.10
Conflict-of-interest disclosure: The author declares no competing financial interests.