Key Points
Quiescent endothelial cells secrete extracellular vesicles that can be taken up by monocytes to suppress their activation.
MiR-10a is transferred to monocytic cells and inhibits the activation of the proinflammatory nuclear factor κB pathway.
Abstract
The blood contains high concentrations of circulating extracellular vesicles (EVs), and their levels and contents are altered in several disease states, including cardiovascular disease. However, the function of circulating EVs, especially the microRNAs (miRNAs) that they contain, are poorly understood. We sought to determine the effect of secreted vesicles produced by quiescent endothelial cells (ECs) on monocyte inflammatory responses and to assess whether transfer of microRNAs occurs between these cells. We observed that monocytic cells cocultured (but not in contact) with ECs were refractory to inflammatory activation. Further characterization revealed that endothelium-derived EVs (EC-EVs) suppressed monocyte activation by enhancing immunomodulatory responses and diminishing proinflammatory responses. EVs isolated from mouse plasma also suppressed monocyte activation. Importantly, injection of EC-EVs in vivo repressed monocyte/macrophage activation, confirming our in vitro findings. We found that several antiinflammatory microRNAs were elevated in EC-EV–treated monocytes. In particular, miR-10a was transferred to monocytic cells from EC-EVs and could repress inflammatory signaling through the targeting of several components of the NF-κB pathway, including IRAK4. Our findings reveal that ECs secrete EVs that can modulate monocyte activation and suggest that altered EV secretion and/or microRNA content may affect vascular inflammation in the setting of cardiovascular disease.
Introduction
Vascular endothelial cells (ECs) recruit circulating monocytes to regions of vascular injury and/or infection.1 Following their entry into the vessel wall, monocytes differentiate into macrophages, which drive an inflammatory response to neutralize invading pathogens, repair tissue damage, or activate other immune cells.2 Monocyte and macrophage phenotypes are highly heterogeneous and can be dynamically modulated by the microenvironment.3-5 Classical activation promotes a proinflammatory (M1-like) response, which includes the secretion of proinflammatory cytokines and reactive oxygen and nitrogen species, and is driven by exposure to bacterial lipopolysaccharide (LPS) or Th1 cytokines such as interferon-γ.4 Conversely, exposure to Th2 cytokines such as interleukin-4 (IL-4), IL-10, or IL-13 supports alternative activation, which is an immunomodulatory, proangiogenic, and tissue-reparative (M2-like) response that includes the secretion of IL-10 and transforming growth factor β (TGF-β).4 The balance of proinflammatory vs immunomodulatory responses appears to play an important but poorly understood role in cardiovascular pathologies.4,6-8 Both transcriptional pathways (eg, IRF5, nuclear factor κ-light-chain-enhancer of activated B cells [NF-κB], and signal transducers and activators of transcription9 ) and posttranscriptional regulators (eg, microRNAs [miRNAs]10-14 ) play key roles in modulating monocyte/macrophage phenotype.
Extracellular vesicles (EVs) of diverse size, composition and cellular origin are abundant in the circulation.15 These EVs include exosomes, microparticles (MPs) and apoptotic bodies (ABs). Secreted EVs can be taken up by target cells, and cell-surface and encapsulated EV proteins can modulate cellular signaling pathways in the recipient cell.16 In addition, microRNAs are packaged into EVs and can alter target gene expression in recipient cells.17 For example, ECs secrete EV-encapsulated miRNAs that can be taken up by smooth muscle cells in vitro.18 The abundance and type of EVs as well as their contents can vary during disease progression, and this has provided an impetus to measure these parameters as circulating biomarkers.15,19 However, the functional consequences of these EV alterations are poorly understood. In inflammatory conditions, the levels of circulating MPs and ABs are highly elevated.15,20 MPs drive EC and monocyte activation and promote chronic vascular inflammatory diseases, such as atherosclerosis, and can be quantified and used as an independent risk factor for adverse cardiovascular events.20 In contrast, ABs derived from ECs have been shown to promote vascular repair and inhibit atherogenesis through their transfer of miR-126 to recipient ECs.21 The role of EVs secreted from quiescent ECs and their contribution to vascular homeostasis are still poorly understood.
miRNAs have been implicated as key regulators of inflammatory signaling pathways in ECs and monocytes/macrophages.22,23 Several miRNAs that are highly expressed in ECs are known to inhibit the proinflammatory NF-κB transcriptional pathway. For example, miR-146a targets adaptor proteins to limit NF-κB signaling,24 and miR-10a represses the expression of proteins that destabilize IκB.25 Finally, miR-181b represses importin-α3 to inhibit NF-κB nuclear import.26 These miRNAs likely play a cooperative role in suppressing EC activation. In the current study, we have assessed whether ECs can modulate myeloid inflammatory responses through secretion of EVs containing antiinflammatory miRNAs. We find that EVs secreted from unstimulated (ie, quiescent) ECs have potent anti-inflammatory properties in vitro and in vivo, and this appears to be due in part to the transfer of antiinflammatory miRNAs, including miR-10a, to recipient monocytes/macrophages. Our studies suggest that circulating EVs and the miRNAs that they contain may have a significant impact on the responsiveness of monocytes/macrophages to inflammatory mediators and that alterations to EVs may impact cardiovascular disease progression.
Materials and methods
Cell culture, coculture experiments, and treatments
Detailed methodology can be found in supplemental Methods (available on the Blood Web site).
Isolation and characterization of EVs
After 48-hour culture of confluent monolayers of human umbilical vein ECs (HUVECs) or human coronary artery ECs (CAECs), culture medium was collected and precleared by centrifugation at 400g for 5 minutes, then 2000g for 20 min to eliminate dead cells and cellular debris. The supernatant was then ultracentrifuged at 120 000g for 120 minutes at 4°C, followed by washing of the EV pellet with phosphate-buffered saline (PBS) at 120 000g for 120 minutes at 4°C (Optima L-100XP Ultracentrifuge, Beckman Coulter). The EV pellet was resuspended in PBS and stored at −80°C. Protein content of EVs was used to normalize for EV quantity between experiments using Pierce microplate BCA protein assay kit (Thermo Scientific). To isolate circulating EVs, mouse blood was collected via cardiac puncture and transferred to EDTA-containing tubes. Plasma was isolated from the blood by centrifugation at 1500g for 10 minutes at 4°C to remove blood cells, then the supernatant was centrifuged at 3000g for 15 minutes at 4°C to remove platelets and cell debris. EVs from 100 µL of plasma were isolated using ExoQuick Precipitation Solution (#EXOQ5A-1, System Biosciences), according to the manufacturer’s recommendations, and resuspended in 50 µL of PBS. EVs were characterized by nanoparticle analysis (as in Dragovic et al27 ; see supplemental Methods for details).
Transfection of cells with siRNA, miRNA mimics, miRNA inhibitors or plasmids, and electroporation of EC-EVs with miR-39 mimic
Detailed methodology can be found in supplemental Methods.
Cloning of luciferase constructs and luciferase assays
Detailed methodology can be found in supplemental Methods.
ELISA
Quantification of IL-12p40 was performed on 50 to 100 μL (of 1 mL total) of THP-1 cell or primary monocyte culture supernatants using a Quantikine enzyme-linked immunosorbent assay (ELISA) kit (DP400, R&D Systems), according to the manufacturer’s recommendations.
Western blotting
Western blots were performed as described previously24 using antibodies directed to IRAK4 (Sigma-Aldrich, SAB3500304), IRF5 (Santa Cruz, sc-390364), CD63 (Santa Cruz, sc-5275), or glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz, sc-47724).
miRNA arrays
miRNA expression was measured in untreated or EC-EV–treated THP-1 cells (10 μg/mL of EC-EVs, 24 hours) using QuantiMir technology (MicroRNA qPCR Array, #RA660A-1) from Systems Biosciences, according to the manufacturer’s recommendations.
Real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR)
In vivo experiments
All animal protocols were approved by the animal care committee at the University Health Network (Toronto) and the Institute for Cardiovascular Prevention (Munich). Peritonitis was induced in C57BL/6 mice (3-4 months of age) with 1 mL of 4% thioglycollate injected intraperitoneally (IP)29 (Sigma-Aldrich). On day 3, EC-EVs (60 µg in 500 µL PBS) or PBS was injected into the peritoneum, and 24 hours later, mice were injected IP with LPS (5 mg/kg) for 2 hours. Peritoneal leukocytes were harvested by lavage.
Statistical analyses
Unless otherwise indicated, data represent the mean of at least 3 independent experiments and error bars represent the standard error of the mean (SEM). Pairwise comparisons were made using a Student t test. Comparison of three or more groups was performed using a 1-way analysis of variance (ANOVA) with Newman-Keuls post-hoc test. A p-value of less than 0.05 was considered to be statistically significant. In all figures, *, **, and *** represent P < .05, < .01, and < .001, respectively.
Results
ECs suppress monocytic proinflammatory responses through a process that does not require direct cell-to-cell contact
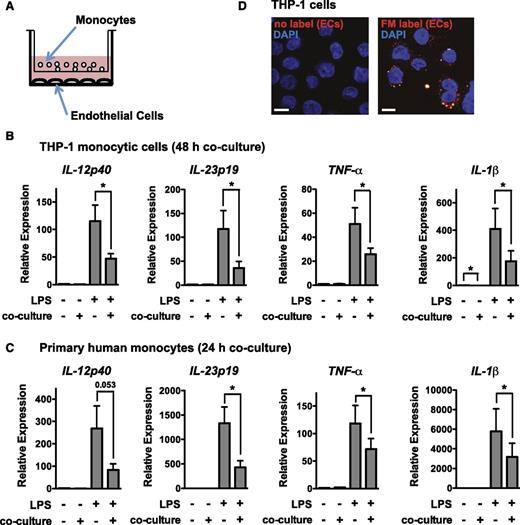
To assess cellular communication between ECs and monocytes, we established a coculture system where HUVECs were cultured together with the human monocytic cell line THP-1 or primary human monocytes, separated by a 1-μm transwell filter (Figure 1A). Following monoculture or coculture, THP-1 cells or primary monocytes were removed from transwell inserts and activated by LPS stimulation. Coculture suppressed monocyte activation, as assessed by expression of IL-12p40, IL-23p19, IL-1β, and TNF-α (Figure 1B-C). We considered whether ECs secrete EVs that are taken up by monocytes. Confocal analysis revealed that THP-1 cells cocultured with membrane-labeled ECs took up the label, demonstrating transfer of membranous vesicles from ECs to monocytic cells (Figure 1D).
Coculture with ECs suppresses monocyte activation in a contact-independent manner. (A) Schematic of coculture of monocytes (THP-1 or primary monocytes) with ECs (HUVECs) using a transwell apparatus (1 μm pore size). (B) Coculture of THP-1 monocytic cells with ECs for 48 hours results in a suppressed responsiveness to a 2-hour LPS stimulation, as assessed by qRT-PCR analysis of proinflammatory genes (normalized to GAPDH). Data are relative to gene expression in THP-1 cells grown in EC medium in monoculture. Shown is the mean ± SEM of 6 independent experiments. (C) Coculture of primary monocytes with ECs for 24 hours results in suppressed LPS responsiveness, as assessed by qRT-PCR analysis of proinflammatory genes (normalized to GAPDH). Shown is the mean ± SEM of 8 independent experiments. (D) THP-1 monocytic cells cocultured with membrane-labeled ECs for 24 hours take up the label. Shown is a representative confocal image. The cell nucleus is stained with 4,6 diamidino-2-phenylindole (DAPI). Scale bar, 10 μm.
Coculture with ECs suppresses monocyte activation in a contact-independent manner. (A) Schematic of coculture of monocytes (THP-1 or primary monocytes) with ECs (HUVECs) using a transwell apparatus (1 μm pore size). (B) Coculture of THP-1 monocytic cells with ECs for 48 hours results in a suppressed responsiveness to a 2-hour LPS stimulation, as assessed by qRT-PCR analysis of proinflammatory genes (normalized to GAPDH). Data are relative to gene expression in THP-1 cells grown in EC medium in monoculture. Shown is the mean ± SEM of 6 independent experiments. (C) Coculture of primary monocytes with ECs for 24 hours results in suppressed LPS responsiveness, as assessed by qRT-PCR analysis of proinflammatory genes (normalized to GAPDH). Shown is the mean ± SEM of 8 independent experiments. (D) THP-1 monocytic cells cocultured with membrane-labeled ECs for 24 hours take up the label. Shown is a representative confocal image. The cell nucleus is stained with 4,6 diamidino-2-phenylindole (DAPI). Scale bar, 10 μm.
Endothelium-derived extracellular vesicles (EC-EVs) suppress monocyte activation and polarize monocyte responses to LPS
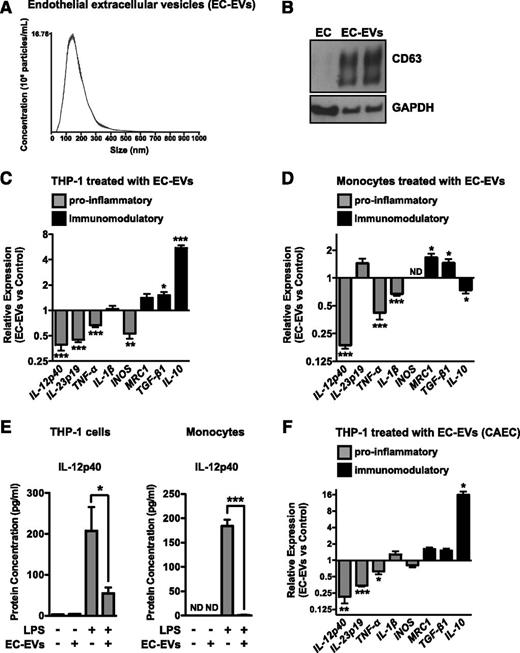
To test whether EC-EVs are involved in suppressing monocyte activation, we isolated EVs from EC culture medium using ultracentrifugation. Nanoparticle analysis demonstrated that the isolated EVs were ∼50 to 300 nm in size (mode = 142 ± 2.6 nm, Figure 2A), consistent with the size of exosomes or small MPs. Western blot analysis revealed that EC-EVs expressed high levels of CD63, an exosomal marker (Figure 2B). To determine the effect of EC-EVs on monocytic cell activation, we added purified EC-EVs to THP-1 monocytic cells for 18 hours, followed by stimulation with LPS. EC-EVs dose-dependently suppressed the activation of proinflammatory genes (eg, IL-12p40, IL-23p19, TNF-α, and IL-1β) while simultaneously enhancing markers of alternative activation or immunomodulatory responses (eg, IL-10, MRC1, and TGF-β) (Figure 2C and supplemental Figures 1A and 2A), suggesting that EC-EVs can polarize monocyte responses. Addition of EC-EVs to primary monocytes also suppressed a subset of proinflammatory genes while enhancing expression of MRC-1 and TGF-β (Figure 2D and supplemental Figure 2B). The suppression of IL-12p40 secretion from THP-1 cells and primary monocytes was confirmed by ELISA (Figure 2E). Although addition of EC-EVs polarized the basal expression of proinflammatory/immunomodulatory markers in unactivated THP-1 cells (supplemental Figure 1B), this was less pronounced in primary monocytes (supplemental Figure 1C). EC-EVs isolated from human CAECs also modulated monocytic cell activation and polarization in response to LPS stimulation, suggesting that this effect is not limited to HUVEC-derived EVs (Figure 2F).
ECs secrete extracellular vesicles (EC-EVs) that inhibit monocyte activation and modulate polarization. (A) Nanoparticle analysis of EVs isolated from HUVEC media by ultracentrifugation. A representative experiment of 3 is shown. The mode particle size is 142 ± 2.6 nm. (B) Western blotting of the exosomal marker, CD63, in lysates from ECs and isolated EC-EVs (2 independent preparations). (C) EC-EVs (10 μg/mL) isolated from HUVECs suppress the activation of THP-1 monocytic cells by LPS (as assessed by qRT-PCR of proinflammatory genes) and promote their polarization (as assessed by qRT-PCR of immunomodulatory markers); n = 5. (D) HUVEC-derived EC-EVs (10 μg/mL) polarize the response of primary monocytes to LPS treatment. ND, not detected. n = 5. (E) Protein levels of IL-12p40 in the medium of EC-EV–treated THP-1 cells and primary monocytes, as measured by ELISA. Cells were either unstimulated or treated with LPS for 8 hours. n = 5. (F) EC-EVs isolated from human CAECs polarize monocyte activation in response to LPS treatment. n = 3.
ECs secrete extracellular vesicles (EC-EVs) that inhibit monocyte activation and modulate polarization. (A) Nanoparticle analysis of EVs isolated from HUVEC media by ultracentrifugation. A representative experiment of 3 is shown. The mode particle size is 142 ± 2.6 nm. (B) Western blotting of the exosomal marker, CD63, in lysates from ECs and isolated EC-EVs (2 independent preparations). (C) EC-EVs (10 μg/mL) isolated from HUVECs suppress the activation of THP-1 monocytic cells by LPS (as assessed by qRT-PCR of proinflammatory genes) and promote their polarization (as assessed by qRT-PCR of immunomodulatory markers); n = 5. (D) HUVEC-derived EC-EVs (10 μg/mL) polarize the response of primary monocytes to LPS treatment. ND, not detected. n = 5. (E) Protein levels of IL-12p40 in the medium of EC-EV–treated THP-1 cells and primary monocytes, as measured by ELISA. Cells were either unstimulated or treated with LPS for 8 hours. n = 5. (F) EC-EVs isolated from human CAECs polarize monocyte activation in response to LPS treatment. n = 3.
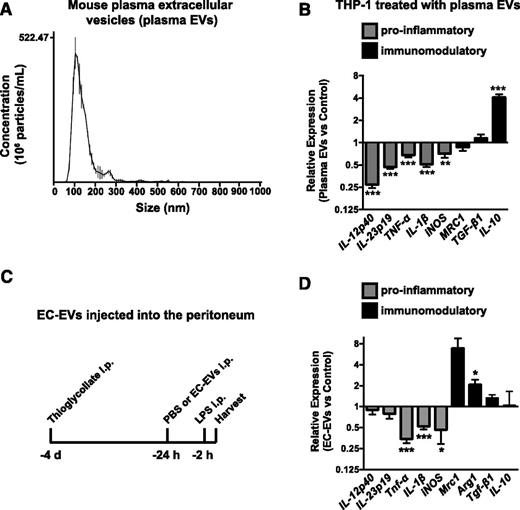
We next sought to determine whether circulating EVs have anti-inflammatory properties in vivo. To this end, we isolated EVs from the plasma of young, healthy mice (Figure 3A). Plasma EVs have both EC and non-EC origins (eg, red blood cells, leukocytes, and platelets). However, due to the lack of cell-of-origin markers on exosomes, the relative contribution of these diverse cell types to plasma EV pools cannot currently be determined. Quantification of EVs in plasma by nanoparticle analysis revealed that there were ∼1.8 × 1011 (±0.33 × 1011) particles/mL. Importantly, our in vitro experiments utilizing EVs isolated from ECs used∼2.1 × 1010 (±0.29 × 1010) particles/mL, suggesting that this is not a supraphysiological dose of EVs. Similar to EC-EVs, addition of plasma EVs to THP-1 cells ex vivo suppressed induction of proinflammatory genes while simultaneously activating IL-10 expression in response to LPS (Figure 3B) but had only a modest effect on these genes in unactivated cells (supplemental Figure 3A).
EVs isolated from mouse plasma suppress monocytic cell activation and EC-EVs suppress peritoneal leukocyte activation in vivo. (A) Nanoparticle analysis of EVs isolated from mouse plasma by ExoQuick isolation. A representative experiment of 4 is shown. The mode particle size is 112 ± 5.5 nm. (B) EVs isolated from mouse plasma suppress THP-1 monocytic-cell activation in response to LPS treatment. n = 9. (C) Peritonitis was established by IP injection of thioglycollate for 3 days. PBS or HUVEC-derived EC-EVs (60 μg) were injected into the peritoneum and the response to LPS treatment (IP, 2 hours) was assessed in peritoneal leukocytes the following day by qRT-PCR analysis. (D) The levels of proinflammatory genes (Tnf-α, IL-1β, and iNOS) are suppressed by EC-EV injection, whereas immunomodulatory genes are elevated. n = 7.
EVs isolated from mouse plasma suppress monocytic cell activation and EC-EVs suppress peritoneal leukocyte activation in vivo. (A) Nanoparticle analysis of EVs isolated from mouse plasma by ExoQuick isolation. A representative experiment of 4 is shown. The mode particle size is 112 ± 5.5 nm. (B) EVs isolated from mouse plasma suppress THP-1 monocytic-cell activation in response to LPS treatment. n = 9. (C) Peritonitis was established by IP injection of thioglycollate for 3 days. PBS or HUVEC-derived EC-EVs (60 μg) were injected into the peritoneum and the response to LPS treatment (IP, 2 hours) was assessed in peritoneal leukocytes the following day by qRT-PCR analysis. (D) The levels of proinflammatory genes (Tnf-α, IL-1β, and iNOS) are suppressed by EC-EV injection, whereas immunomodulatory genes are elevated. n = 7.
To determine if HUVEC-derived EVs can suppress inflammation in vivo, we used a mouse peritonitis model, where thioglycollate was injected into the peritoneum to induce monocyte recruitment and macrophage differentiation.30 We then injected PBS or EC-EVs into the peritoneum and assessed the inflammatory response of the recruited leukocytes to LPS challenge (injected IP) the following day (Figure 3C). EVs have previously been used in cross-species experiments with no obvious immunogenic complications.31,32 Importantly, neutrophil and macrophage markers were unchanged in response to EC-EV injection into the peritoneum (supplemental Figure 3B). In LPS-stimulated mice, EC-EVs suppressed the induction of proinflammatory genes, such as Tnf-α, iNOS, and IL-1β, while significantly enhancing the expression of Arg1, a murine marker of immunomodulatory responses (Figure 3D). The expression of proinflammatory/immunomodulatory markers in non–LPS-stimulated mice was largely unaffected (supplemental Figure 3C). IL-12p40, IL-23p19, and IL-10 were expressed at extremely low levels in unactivated and LPS-injected mice (data not shown), and no changes were observed in their expression (Figure 3D and supplemental Figure 3C).
EC-EVs inhibit NF-κB signaling and IRF5 expression
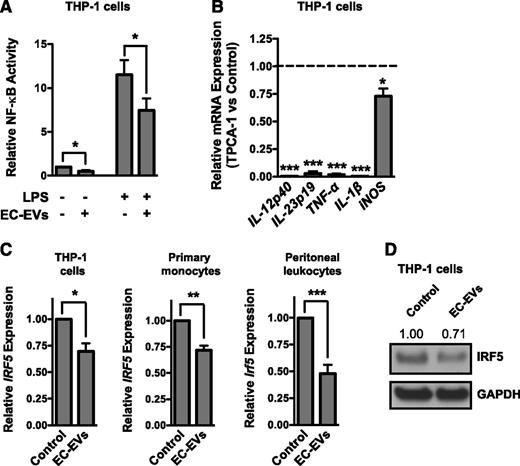
Many proinflammatory genes require NF-κB signaling for their induction.33,34 We therefore assessed the effect of EC-EVs on activity of an NF-κB-dependent luciferase reporter. Addition of EC-EVs to THP-1 cells suppressed the basal and LPS-stimulated activity of the reporter (Figure 4A). We next assessed whether inhibition of NF-κB signaling could recapitulate the effects of EC-EVs. Indeed, the induction of proinflammatory genes in LPS-stimulated THP-1 monocytic cells was suppressed by the IKK inhibitor TPCA-1 (Figure 4B). However, LPS induction of immunomodulatory genes, such as TGF-β and IL-10, was not enhanced in monocytic cells treated with the NF-κB inhibitor (data not shown). IRF5 is a marker of monocyte/macrophage polarization that has previously been implicated in the induction of proinflammatory genes while simultaneously suppressing immunomodulatory genes.6,35 Consistent with the polarization effects of EC-EVs, we found that IRF5 expression was significantly inhibited by EC-EV treatment of THP-1 cells or primary monocytes (Figure 4C-D). Additionally, the expression of Irf5 was reduced in peritoneal leukocytes exposed to EC-EVs in vivo (Figure 4C). EC-EVs can therefore suppress transcriptional pathways (ie, NF-κB and IRF5) that are known to be indicative of polarized monocyte/macrophage activation.
EC-EVs suppress the induction of NF-κB and repress the expression of IRF5. (A) EC-EV–treated THP-1 monocytic cells have reduced basal and LPS-stimulated NF-κB reporter activity. n = 4. (B) THP-1 cells pretreated with the NF-κB inhibitor, TPCA-1 (10 μM), have reduced induction of proinflammatory genes in response to LPS stimulation. n = 4. (C) EC-EV–treated THP-1 cells or primary monocytes have reduced levels of IRF5 mRNA as assessed by qRT-PCR. n = 5. Peritoneal leukocytes isolated from EC-EV–injected mice have reduced levels of Irf5 mRNA compared with PBS-injected controls. n = 7. (D) Western blot of IRF5 in EC-EV–treated THP-1 cells. Densitometry is indicated above. Shown is a representative result of 4 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
EC-EVs suppress the induction of NF-κB and repress the expression of IRF5. (A) EC-EV–treated THP-1 monocytic cells have reduced basal and LPS-stimulated NF-κB reporter activity. n = 4. (B) THP-1 cells pretreated with the NF-κB inhibitor, TPCA-1 (10 μM), have reduced induction of proinflammatory genes in response to LPS stimulation. n = 4. (C) EC-EV–treated THP-1 cells or primary monocytes have reduced levels of IRF5 mRNA as assessed by qRT-PCR. n = 5. Peritoneal leukocytes isolated from EC-EV–injected mice have reduced levels of Irf5 mRNA compared with PBS-injected controls. n = 7. (D) Western blot of IRF5 in EC-EV–treated THP-1 cells. Densitometry is indicated above. Shown is a representative result of 4 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
EC-EVs transfer miRNAs to recipient monocytic cells
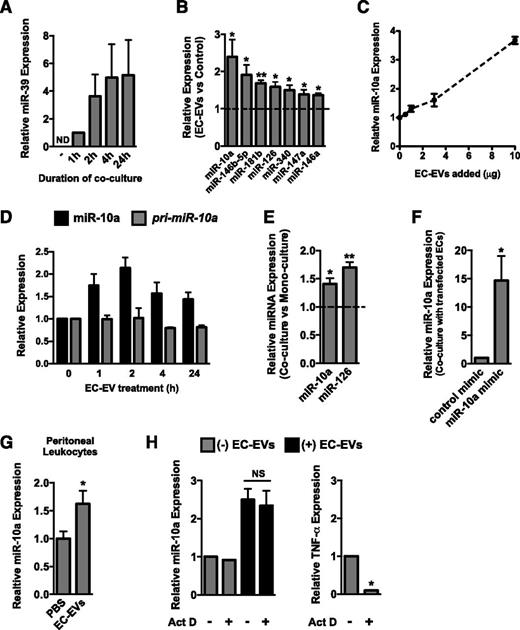
Because EVs can transfer their miRNA contents to recipient cells in other cellular contexts,17 we tested whether miRNAs could be transferred from ECs to monocytic cells in the absence of cell-cell contact. Indeed, transfection of the Caenorhabditis elegans–specific miRNA, miR-39, into ECs resulted in a time-dependent accumulation of miR-39 in cocultured THP-1 cells (Figure 5A). To establish that miRNAs can be transferred from EC-EVs to peritoneal leukocytes in vivo, we electroporated miR-39 into EC-EVs and injected these miR-39–loaded EVs into the peritoneum 3 days following thioglycollate challenge. Isolation of peritoneal leukocytes, spleen, bone marrow, and peripheral blood 24 hours after EV injection revealed that EVs (as assessed by miR-39 expression) were primarily taken up by peritoneal cells (supplemental Figure 3D). To identify the repertoire of miRNAs that might be transferred from ECs to monocytic cells, we performed quantitative PCR (qPCR) miRNA arrays on THP-1 cells treated with EC-EVs and compared this to untreated cells (data not shown). qRT-PCR was used to validate several of the miRNAs identified as differentially expressed (Figure 5B). As anticipated, the EC-enriched miRNA miR-126-3p, which has been implicated in the suppression of inflammation,36,37 was elevated in EC-EV–treated monocytic cells. Interestingly, several other microRNAs with a known role in the repression of inflammatory signaling (ie, miR-10a, miR-146a/b, miR-147a, and miR-181b24-26,38,39 ) were also increased in the recipient THP-1 cells, most notably miR-10a (Figure 5B). The majority of these miRNAs were also elevated in EC-EV–treated primary monocytes (supplemental Figure 4A). MiR-10a, miR-181b, and miR-126-3p were abundant in HUVECs and EC-EVs (supplemental Figure 4B-C). Interestingly, miR-10a was significantly enriched in EVs compared with the cells of origin, whereas miR-126-3p and miR-181b were not (supplemental Figure 4D). This suggests that miR-10a may be selectively loaded into EC-EVs. Importantly, a similar repertoire of miRNAs was contained in EVs isolated from CAECs (supplemental Figure 4E), and these miRNAs were also present in EVs isolated from mouse plasma (supplemental Figure 4F).
MiR-10a is transferred from EC-EVs to monocytic cells. (A) Overexpression of the C elegans–specific miRNA, miR-39, in ECs results in a time-dependent accumulation of this miRNA in cocultured THP-1 cells (in the absence of cell-cell contact). MiR-39 expression level after 1-hour coculture was set to 1 because miR-39 was not detectable (ND) by qRT-PCR in THP-1 monoculture. Data are normalized to U6 expression. n = 3. (B) qRT-PCR was used to assess the expression of several miRNAs, including the EC-enriched miRNA, miR-126-3p, and several anti-inflammatory miRNAs (miR-10a, miR-146a/b, miR-147a, and miR-181b) in THP-1 monocytic cells treated with EC-EVs for 24 hours. Data are normalized to U6 expression. n = 5. (C) Addition of increasing concentrations of EC-EVs results in a dose-dependent increase in miR-10a in treated THP-1 cells. n = 2. (D) Kinetics of mature miR-10a and pri-miR-10a expression after treatment of THP-1 cells with EC-EVs (10 μg/mL). n = 2. (E) Levels of miR-10a and miR-126-3p are increased in THP-1 cells cocultured with ECs for 24 hours. n = 4. (F) Overexpression of miR-10a in ECs results in an increase in miR-10a expression in cocultured THP-1 cells. n = 4. (G) miR-10a expression is elevated in peritoneal leukocytes isolated from LPS-stimulated mice injected with EC-EVs compared with PBS injection control. n = 5. (H) EC-EV–dependent induction of miR-10a is resistant to actinomycin D (Act D) pretreatment of THP-1 cells, suggesting a transfer from EVs. Expression of TNF-α, a short-lived transcript, is reduced in actinomycin D–treated cells. n = 3.
MiR-10a is transferred from EC-EVs to monocytic cells. (A) Overexpression of the C elegans–specific miRNA, miR-39, in ECs results in a time-dependent accumulation of this miRNA in cocultured THP-1 cells (in the absence of cell-cell contact). MiR-39 expression level after 1-hour coculture was set to 1 because miR-39 was not detectable (ND) by qRT-PCR in THP-1 monoculture. Data are normalized to U6 expression. n = 3. (B) qRT-PCR was used to assess the expression of several miRNAs, including the EC-enriched miRNA, miR-126-3p, and several anti-inflammatory miRNAs (miR-10a, miR-146a/b, miR-147a, and miR-181b) in THP-1 monocytic cells treated with EC-EVs for 24 hours. Data are normalized to U6 expression. n = 5. (C) Addition of increasing concentrations of EC-EVs results in a dose-dependent increase in miR-10a in treated THP-1 cells. n = 2. (D) Kinetics of mature miR-10a and pri-miR-10a expression after treatment of THP-1 cells with EC-EVs (10 μg/mL). n = 2. (E) Levels of miR-10a and miR-126-3p are increased in THP-1 cells cocultured with ECs for 24 hours. n = 4. (F) Overexpression of miR-10a in ECs results in an increase in miR-10a expression in cocultured THP-1 cells. n = 4. (G) miR-10a expression is elevated in peritoneal leukocytes isolated from LPS-stimulated mice injected with EC-EVs compared with PBS injection control. n = 5. (H) EC-EV–dependent induction of miR-10a is resistant to actinomycin D (Act D) pretreatment of THP-1 cells, suggesting a transfer from EVs. Expression of TNF-α, a short-lived transcript, is reduced in actinomycin D–treated cells. n = 3.
To determine whether miR-10a was directly transferred to monocytic cells, we used several independent approaches. Addition of increasing concentrations of EC-EVs resulted in a dose-dependent increase of miR-10a in THP-1 cells (Figure 5C). Importantly, miR-10a levels rapidly increased in monocytic cells treated with EC-EVs, but the primary miR-10a transcript was not induced, suggesting a lack of transcriptional induction (Figure 5D). Coculture of THP-1 monocytic cells with ECs resulted in increased levels of miR-10a and miR-126-3p in monocytic cells (Figure 5E), suggesting a transfer from ECs, where these miRNAs are highly expressed. In addition, transfection of miR-10a mimic into ECs resulted in an increase in miR-10a in cocultured monocytic cells (Figure 5F). Furthermore, miR-10a levels were elevated in peritoneal cells isolated from mice injected IP with EC-EVs compared with PBS injection (Figure 5G). To establish that transcription is indeed not required for miR-10a induction, we pretreated THP-1 cells with the transcriptional inhibitor actinomycin D. EC-EV–mediated induction of miR-10a was not affected by actinomycin D treatment (Figure 5H). However, expression of the short-lived transcript, TNF-α, was reduced in actinomycin D–treated cells, demonstrating the effectiveness of the dose used (Figure 5H). Taken together, these data provide evidence that miR-10a is directly transferred from EC-EVs to monocytic cells.
MiR-10a, miR-126, and miR-181b can suppress proinflammatory monocytic cell activation
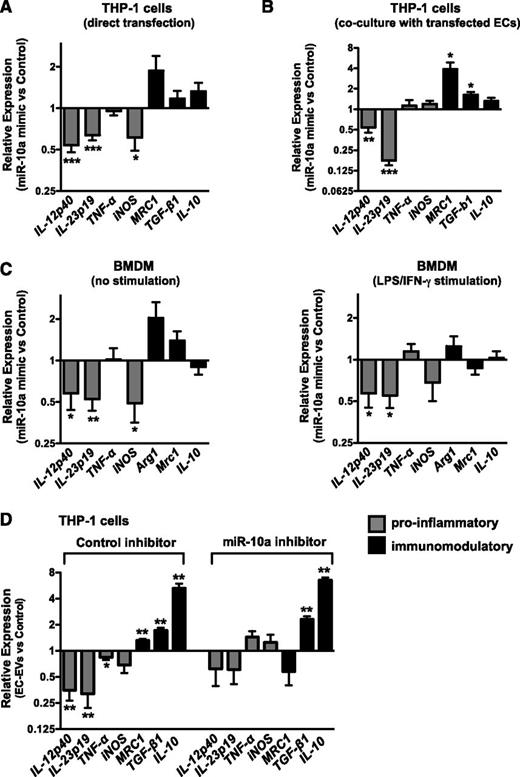
Because several miRNAs were elevated in EC-EV–treated monocytic cells (Figure 5B), we sought to determine whether overexpression of any one of these miRNAs could recapitulate the EC-EV effects on the polarization of monocyte activation. We individually overexpressed miR-10a, miR-126, miR-146a, miR-146b, miR-181b, or miR-340 in THP-1 monocytic cells (Figure 6A and supplemental Figure 5A) or in ECs cocultured with THP-1 cells (Figure 6B and supplemental Figure 5B). Interestingly, overexpression of miR-10a, miR-126, or miR-181b could suppress the induction of proinflammatory genes in THP-1 cells stimulated with LPS, whereas miR-146a/b and miR-340 did not have significant effects. Modest elevation of immunomodulatory genes (which did not consistently reach statistical significance) was observed with miR-10a transfection. Overexpression of miR-10a in primary BMDMs also suppressed proinflammatory gene expression in both unstimulated and LPS/interferon-γ–stimulated cells (Figure 6C). To further assess the role of endogenous and EC-EV–derived miR-10a in monocyte activation, we inhibited miR-10a in THP-1 cells prior to treating them with EC-EVs. While EC-EVs were able to suppress the induction of proinflammatory genes in control inhibitor-treated monocytic cells, the effect of EC-EVs on these genes was attenuated in miR-10a–inhibited monocytic cells (Figure 6D). In addition, the induction of the immunomodulatory marker MRC1 was abrogated in miR-10a–inhibited monocytic cells, although the induction of IL-10 and TGF-β1 was unaffected (Figure 6D). Taken together, these results suggest that miR-10a contributes to the suppression of proinflammatory genes and may modestly affect immunomodulatory responses.
miR-10a suppresses monocyte/macrophage activation. (A) MiR-10a overexpression in THP-1 monocytic cells suppresses the induction of proinflammatory genes in response to LPS stimulation, as assessed by qRT-PCR. n = 6. (B) MiR-10a overexpression in ECs suppresses the induction of proinflammatory genes in cocultured THP-1 cells stimulated with LPS. Values are expressed relative to cocultured cells transfected with control mimic, and the suppressive effective of miR-10a overexpression is therefore in addition to the effect of EC coculture on monocytic cell activation. n = 6. (C) Overexpression of miR-10a in mouse bone marrow–derived macrophages (BMDMs) suppresses the expression of proinflammatory genes. Shown are unstimulated BMDMs (left) or BMDMs treated with LPS/interferon-γ (right). n = 6 and n = 5, respectively. (D) Inhibiting miR-10a in THP-1 monocytic cells negates the EC-EV–dependent downregulation of proinflammatory genes and affects the expression of the immunomodulatory marker MRC1 in LPS-stimulated cells. Data are expressed relative to cells transfected with control inhibitor with no EC-EVs. n = 6.
miR-10a suppresses monocyte/macrophage activation. (A) MiR-10a overexpression in THP-1 monocytic cells suppresses the induction of proinflammatory genes in response to LPS stimulation, as assessed by qRT-PCR. n = 6. (B) MiR-10a overexpression in ECs suppresses the induction of proinflammatory genes in cocultured THP-1 cells stimulated with LPS. Values are expressed relative to cocultured cells transfected with control mimic, and the suppressive effective of miR-10a overexpression is therefore in addition to the effect of EC coculture on monocytic cell activation. n = 6. (C) Overexpression of miR-10a in mouse bone marrow–derived macrophages (BMDMs) suppresses the expression of proinflammatory genes. Shown are unstimulated BMDMs (left) or BMDMs treated with LPS/interferon-γ (right). n = 6 and n = 5, respectively. (D) Inhibiting miR-10a in THP-1 monocytic cells negates the EC-EV–dependent downregulation of proinflammatory genes and affects the expression of the immunomodulatory marker MRC1 in LPS-stimulated cells. Data are expressed relative to cells transfected with control inhibitor with no EC-EVs. n = 6.
MiR-10a represses NF-κB signaling
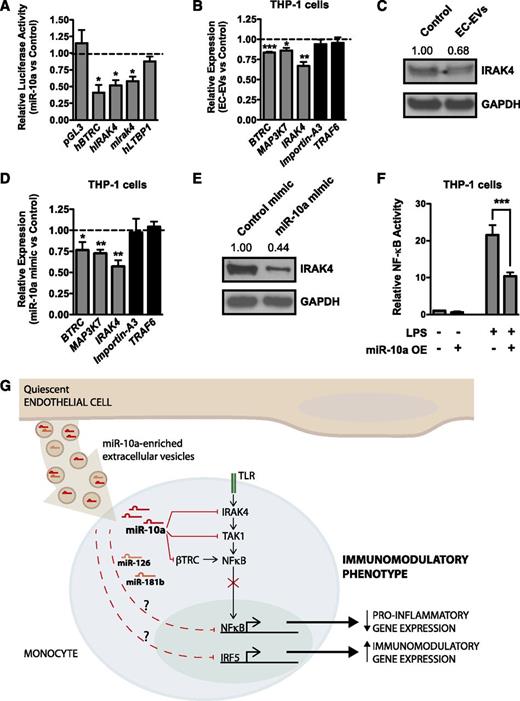
MiR-10a was previously shown to suppress NF-κB signaling in ECs through the repression of β-TRC and MAP3K7/TAK1, effector molecules required for IκB degradation and hence NF-κB activity.25 Importantly, monocytic cells treated with EC-EVs have reduced NF-κB activity (Figure 4A). We identified IRAK4, a known regulator of NF-κB signaling, and latent TGF-β binding protein (LTBP1), a suppressor of TGF-β signaling, as novel potential miR-10a target genes (supplemental Figure 6). We first assessed whether IRAK4 or LTBP1 were targets of miR-10a by cloning a portion of the 3′ untranslated regions (UTRs) of these genes downstream of a luciferase open reading frame (Figure 7A). As previously demonstrated,25 luciferase constructs containing the BTRC 3′ UTR were repressed by miR-10a overexpression. Although LTBP1 was not regulated by miR-10a, the 3′ UTRs of both mouse and human IRAK4 were regulated by miR-10a (Figure 7A). We next assessed whether EC-EVs were able to suppress miR-10a targets in recipient monocytic cells. BTRC, MAP3K7, and IRAK4 messenger RNA (mRNA) levels and IRAK4 protein levels were decreased in EC-EV–treated THP-1 cells. However, targets of miR-146a/b (ie, TRAF6) and miR-181b (ie, Importin-A3) were unaffected, at least at the mRNA level (Figure 7B-C). In addition, overexpression of miR-10a in THP-1 monocytic cells repressed BTRC, MAP3K7, and IRAK4 mRNA and IRAK4 protein expression (Figure 7D-E). Because IRAK4, β-TRC, and MAP3K7 act upstream of NF-κB activation, we assessed NF-κB reporter activity in monocytic cells transfected with miR-10a mimic. As anticipated, miR-10a overexpression suppressed NF-κB activity (Figure 7F). Taken together, these findings delineate a pathway whereby transfer of miR-10a from EC-EVs to monocytes can repress a network of genes involved in NF-κB signaling to suppress the activation of proinflammatory genes (Figure 7G).
MiR-10a negatively regulates NF-κB signaling. (A) Luciferase assays in bovine aortic ECs. BTRC, a known miR-10a target, is included as a positive control. Overexpression of miR-10a suppresses the activity of mouse and human IRAK4 3′ UTR–containing luciferase constructs, but LTBP1 (another miR-10a predicted target gene) is not suppressed. n = 3. (B) EC-EV treatment suppresses BTRC and MAP3K7 (known miR-10a target genes), as well as IRAK4 mRNA expression in LPS-stimulated (2 hours) THP-1 cells, whereas effects on known miR-146a (TRAF6) or miR-181b (Importin-A3) targets are not observed. n = 4. (C) Western blotting confirms that IRAK4 protein is suppressed by EC-EV treatment. Densitometry is indicated above. A representative experiment of 4 is shown. (D) MiR-10a overexpression (OE) in THP-1 monocytic cells represses BTRC, MAP3K7, and IRAK4 mRNA levels. n = 4. (E) MiR-10a overexpression in THP-1 cells represses IRAK4 protein as assessed by western blot. Densitometry is indicated above. A representative experiment of 3 is shown. (F) NF-κB activity is repressed in THP-1 cells overexpressing miR-10a. Shown is a representative experiment of 4 with triplicate determinations. (G) Model of how ECs suppress monocytic inflammatory responses and promote an immunomodulatory phenotype through the secretion of EC-EVs that contain antiinflammatory miRNAs, including miR-10a. Suppression of NF-κB signaling is mediated in part by the targeting of IRAK4, TAK1/MAP3K7, and β-TRC by miR-10a, whereas IRF5 is downregulated by EC-EVs in an miR-10a–independent manner (data not shown). MiR-126 and miR-181b (which are present in EC-EVs) can suppress proinflammatory responses when overexpressed in monocytic cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
MiR-10a negatively regulates NF-κB signaling. (A) Luciferase assays in bovine aortic ECs. BTRC, a known miR-10a target, is included as a positive control. Overexpression of miR-10a suppresses the activity of mouse and human IRAK4 3′ UTR–containing luciferase constructs, but LTBP1 (another miR-10a predicted target gene) is not suppressed. n = 3. (B) EC-EV treatment suppresses BTRC and MAP3K7 (known miR-10a target genes), as well as IRAK4 mRNA expression in LPS-stimulated (2 hours) THP-1 cells, whereas effects on known miR-146a (TRAF6) or miR-181b (Importin-A3) targets are not observed. n = 4. (C) Western blotting confirms that IRAK4 protein is suppressed by EC-EV treatment. Densitometry is indicated above. A representative experiment of 4 is shown. (D) MiR-10a overexpression (OE) in THP-1 monocytic cells represses BTRC, MAP3K7, and IRAK4 mRNA levels. n = 4. (E) MiR-10a overexpression in THP-1 cells represses IRAK4 protein as assessed by western blot. Densitometry is indicated above. A representative experiment of 3 is shown. (F) NF-κB activity is repressed in THP-1 cells overexpressing miR-10a. Shown is a representative experiment of 4 with triplicate determinations. (G) Model of how ECs suppress monocytic inflammatory responses and promote an immunomodulatory phenotype through the secretion of EC-EVs that contain antiinflammatory miRNAs, including miR-10a. Suppression of NF-κB signaling is mediated in part by the targeting of IRAK4, TAK1/MAP3K7, and β-TRC by miR-10a, whereas IRF5 is downregulated by EC-EVs in an miR-10a–independent manner (data not shown). MiR-126 and miR-181b (which are present in EC-EVs) can suppress proinflammatory responses when overexpressed in monocytic cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Here, we demonstrate that quiescent ECs secrete extracellular vesicles (EC-EVs) that have potent antiinflammatory activities. Primary monocytes and THP-1 monocytic cells treated with EC-EVs in vitro and peritoneal leukocytes exposed to EC-EVs in vivo have reduced proinflammatory responses and enhanced immunomodulatory responses, and this is accompanied by suppressed NF-κB signaling and reduced levels of IRF5. The antiinflammatory effects of EC-EVs appear to be attributable in part to the transfer of miR-10a from EC-EVs to monocytes/macrophages. Exogenous miR-10a suppresses NF-κB activity by targeting several components of the NF-κB pathway, including IRAK4, β-TRC, and MAP3K7. In addition, we find that miR-126 and miR-181b are also increased in EC-EV–treated monocytic cells, and overexpression of these miRNAs can suppress monocyte activation. These antiinflammatory miRNAs may act together to suppress monocyte activation. Importantly, these microRNAs are conserved between mice and humans, which may explain why human EC-EVs can potently inhibit the activation of mouse leukocytes. It is likely that proteins or lipids contained in EC-EVs also contribute to their antiinflammatory and immunomodulatory effects.
Monocytes are continually exposed to EVs in the circulation, but little is known regarding the functional consequences of EV uptake. Prior studies have revealed that endothelium-derived MPs, which are present at elevated levels in chronic inflammatory states, are preferentially taken up by monocytes compared with neutrophils and lymphocytes and that exposure to endothelium-derived MPs leads to monocyte activation.40 In contrast, our studies suggest that healthy, quiescent endothelium secretes EVs (exosomes and small MPs) that reduce the magnitude of proinflammatory responses and shifts monocyte activation toward an immunomodulatory response. We have additionally found that these EC-EVs can suppress EC activation (M.-S.N. and J.E.F., unpublished data), suggesting that their protective effects may extend beyond the regulation of monocyte activation. Endothelial dysfunction and activation occur in various chronic cardiovascular disease states,41 and this likely contributes to alterations in the levels and types of EVs (ie, exosomes vs MPs vs ABs) in circulation, as well as modulating their contents, ultimately influencing their function. For example, MP secretion is enhanced from ECs and leukocytes exposed to inflammatory mediators, and these vesicles have been shown to promote monocyte activation, thrombosis, and the dysfunction/activation of ECs during atherogenesis.15,40 Interestingly, we have found that EVs isolated from acutely activated ECs lose some of their antiinflammatory properties (supplemental Figure 7), suggesting that altered EV function may be an early event in vascular inflammatory disease.
Our findings provide a new paradigm for the role of ECs in orchestrating vascular inflammation in cardiovascular disease. We propose that the antiinflammatory activities of EVs that we have observed in healthy mice may be lost during disease pathogenesis, and we anticipate that this can influence the activation state of circulating monocytes. This may in turn influence the type of macrophage response (ie, proinflammatory or immunomodulatory) that occurs in the setting of vascular inflammation. Interestingly, recent studies have revealed that MPs isolated from patients with coronary artery disease contain less miRNA and have defects in their ability to transfer these miRNAs to recipient cells.42 Our findings show that EVs that are constitutively secreted by ECs harbor protective miRNAs that can be transferred to monocytes. We speculate that MPs present in chronic inflammatory conditions may lack this repertoire of miRNAs or they may contain inflammatory mediators (ie, protein or lipid) that override this antiinflammatory effect.
MiR-10a appears to play a critical role in the inflammatory response. For example, circulating levels of miR-10a are reduced in patients suffering from acute pancreatitis,43 and exposure to microbiota can downregulate miR-10a in dendritic cells in an NF-κB–dependent manner.44 Importantly, miR-10a levels are also decreased in atherosclerotic plaque.45 In contrast to the downregulation of miR-10a by inflammatory stimuli, elegant studies have shown that laminar shear stress enhances EC miR-10a expression, whereas disturbed flow (which is accompanied by NF-κB activation) represses miR-10a expression.25 Fang et al demonstrate that miR-10a inhibits NF-κB signaling and EC activation, and speculate that atherosclerosis may develop preferentially in regions of disturbed flow because of a reduction in miR-10a expression.25 In addition to targeting several components of the NF-κB pathway, miR-10a can also directly target the proinflammatory cytokine IL-12p40 in mouse dendritic cells,44 and we provide evidence that IL-12p40 is also suppressed by miR-10a overexpression in monocytic cells. Our findings suggest that miR-10a secreted from ECs inhibits monocyte activation, and therefore, loss of miR-10a in ECs during atherogenesis45 may in turn influence monocyte activation. Taken together, our studies reveal a novel crosstalk between the endothelium and monocytes/macrophages that is mediated via secreted EVs that contain antiinflammatory miRNAs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr C. Boulanger and X. Loyer (INSERM) for insightful discussions. They also thank the Transplantation Core Laboratory (Toronto) for the use of their ultracentrifuge and Dr C. Librach (CReATe Fertility Centre, Toronto) and laboratory personnel, especially A. Azizeddin and P. Mackie, for technical assistance with NanoSight analysis.
This research was supported by a group grant to J.E.F. and A.S. (Canadian Institutes of Health Research [CIHR] MTA-118968, German Federal Ministry of Education and Research 01KU1213A, and German Centre for Cardiovascular Research VD1.2), seed funding from the CIHR Canadian Vascular Network, and an Innovation Grant from the Canadian Cancer Society (#702835). J.E.F. is supported by a Canada Research Chair from CIHR and an Early Researcher Award from the Ontario Ministry of Research and Innovation. M.-S.N. is supported by a fellowship from the Canadian Vascular Network, and H.S.C. is supported by an Ontario Graduate Studentship.
Authorship
Contribution: M.-S.N. designed and performed experiments, analyzed data, and wrote the manuscript; H.S.C., L.T.D., M.N.-J., A.C.L., M.R., and E.B. designed and performed experiments and analyzed data; A.S. supervised M.N.-J. and designed experiments; M.I.C. supervised A.C.L. and M.R. and designed experiments; and J.E.F. supervised M.-S.N., H.S.C., L.T.D., and E.B., designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason E. Fish, Toronto General Research Institute, University Health Network, Toronto Medical Discovery Tower, MaRS Building, 101 College St, 3-308, Toronto, ON M5G 1L7, Canada; e-mail: jason.fish@utoronto.ca.