In this issue of Blood, Schinke et al have identified a higher expression of CXCR2 in myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) stem cells than in normal hematopoietic stem cells (HSCs) and demonstrated preclinical therapeutic proof of principle.1
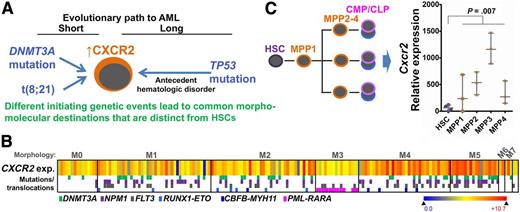
(A) Neoplastic evolution demonstrates convergence, from disparate genetic origins toward a common destination of myeloblasts bearing gross molecular and morphologic similarities. (B) Heatmap shows CXCR2 expression (exp.) in primary AML cells categorized by morphology (M0-M7) (TCGA, n = 171, RNA sequencing). The color coding beneath indicates a subset of contained recurrent mutations and chromosome translocations (some of which are known as first hits). Gene expression by RNA sequencing. (C) Cxcr2 expression is dramatically upregulated in the immediate committed progeny (MPP) of normal HSCs. The implication is that broadly relevant nononcogene therapeutic targets of opportunity such as CXCR2 likely reflect a common theme in neoplastic evolution: transformation in committed progenitors despite origin of the first hit in HSCs or the germline. Box-and-whiskers plot data are expressed as median ± range. P = .007, Mann-Whitney test. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; MMP, multipotent myeloid progenitor (public data E-MTAB-2262).
(A) Neoplastic evolution demonstrates convergence, from disparate genetic origins toward a common destination of myeloblasts bearing gross molecular and morphologic similarities. (B) Heatmap shows CXCR2 expression (exp.) in primary AML cells categorized by morphology (M0-M7) (TCGA, n = 171, RNA sequencing). The color coding beneath indicates a subset of contained recurrent mutations and chromosome translocations (some of which are known as first hits). Gene expression by RNA sequencing. (C) Cxcr2 expression is dramatically upregulated in the immediate committed progeny (MPP) of normal HSCs. The implication is that broadly relevant nononcogene therapeutic targets of opportunity such as CXCR2 likely reflect a common theme in neoplastic evolution: transformation in committed progenitors despite origin of the first hit in HSCs or the germline. Box-and-whiskers plot data are expressed as median ± range. P = .007, Mann-Whitney test. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; MMP, multipotent myeloid progenitor (public data E-MTAB-2262).
The intent driving the idea of “precision medicine,” or the individualization of oncotherapy based on case-by-case cancer genetics, is selective suppression of malignant clones while sparing the normal stem cells needed for health and life—stated simply, a better therapeutic index. Schinke et al identified higher expression of CXCR2 in MDS/AML stem cells than in normal HSCs and demonstrated preclinical therapeutic proof of principle, affirming that molecular targets offering a good therapeutic index are not restricted to mutated oncoproteins. In fact, CXCR2 and other nononcogene cancer molecular addictions could have advantages as targets for therapy, such as near-term drugability1 and broad applicability to a histologic diagnosis. Per the latter, it is noteworthy that high CXCR2 expression is a characteristic of some morphologic subtypes of AML despite different underlying driver mutations (see figure).2 Stated another way, there is convergent neoplastic evolution, from disparate genetic origins toward expansion of myeloblasts bearing gross molecular and morphologic similarities. Why do multiple roads, cobbled by Darwinian selective pressures working for years, lead to similar molecular-morphologic destinations that are distinct from normal HSCs? Here are some clues: “first hits” in the multihit process of leukemogenesis (eg, RUNX1 or DNMT3A mutations) originate in the germline or HSCs (cells of origin) and, accordingly, can be detected in the germline or HSCs.3-5 Subsequent evolution into AML-initiating cells (AML stem cells), however, occurs in lineage-committed daughter cells of these cells of origin, most cleanly documented by the detection of secondary mutations needed for transformation into AML (eg, FLT3 or NPM1 mutations) only in committed progenitors and not in HSCs.3-5 In fact, first hits such as RUNX1 loss-of-function have been shown to manifest their most dramatic phenotypic effects not in HSCs but in committed daughter cells (progenitors) by impeding the differentiation (maturation) that is the natural control on the cell growth and division of this intrinsically MYC-enriched, exponentially expanding compartment.6 In short, transformation into MDS/AML is in a cellular context biologically distinct from that of HSCs,7,8 and CXCR2 and a number of other molecular targets that have been found to discriminate between MDS/AML stem cells and HSCs8 are opportunistic targets, reflecting this compartment shift from stem cells to progenitors that occurs in neoplastic evolution (see figure). The wealth of discriminating molecular targets so created is great news, because there could be many reasons, such as lack of drugability without off-target effects or importance to some other normal function,1 that could undermine the therapeutic index of any one target. This target-rich environment, not restricted to mutated oncoproteins, offers real hope for breakthroughs (or at least improvements in safety and effectiveness) in MDS/AML therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.