Key Points
Mice overexpressing T-bet in T cells show aberrant hematopoiesis of myeloid cells and functional conversion of regional macrophages.
The mice developed a severe PAP-like disease with a hematopoietic disorder resembling the human disease.
Abstract
Although overexpression of T-bet, a master transcription factor in type-1 helper T lymphocytes, has been reported in several hematologic and immune diseases, its role in their pathogenesis is not fully understood. In the present study, we used transgenic model mice (T-bettg/wt and T-bettg/tg) to investigate the effects of T-bet overexpression selectively in T lymphocytes on the development of hematologic and immune diseases. The results showed that T-bet overexpression in T cells spontaneously induced maturation arrest in the mononuclear phagocyte lineage, as well as spontaneous dermatitis and pulmonary alveolar proteinosis (PAP)-like disease in T-bettg/wt and T-bettg/tg mice, respectively. T-bettg/tg alveoli with the PAP phenotype showed remarkable reorganization of alveolar mononuclear phagocyte subpopulations and impaired function, in addition to augmented T-cell infiltration. In addition, PAP development in T-bettg/tg mice was found to be associated with increased migration of myeloid cells from the bone marrow into the peripheral blood. These findings reveal an unexpected link between T-bet overexpression in T lymphocytes and the development of PAP caused by reorganization of mononuclear phagocytes in the lung, and provide new insight into the molecular pathogenesis of secondary PAP accompanied by hematologic disorders.
Introduction
Naïve CD4+ T cells differentiate into various subsets, including type-1 helper (Th1), type-2 helper, type-17 helper, T-follicular helper, and regulatory T cells, in response to specific stimuli provided by cells of the innate immune system, and in response to signals driven by the major histocompatibility complex:peptide complex. The transcription factor T-bet was originally identified as a T-cell transcription factor regulating the Th1 cell differentiation program.1 T-bet not only promotes the expression of IFN-γ, a Th1 cytokine, it also activates CD4+ T cells while suppressing the type-2 helper, type-17 helper, and T-follicular helper cell differentiation programs, and it is also a critical regulator for controlling antimicrobial type 1 inflammatory responses that coordinate gene expression in other immune cells.2 Because of its expression in Th1-type CD4+ T cells, T-bet dysregulation has been implicated in various immunopathological, autoimmune, and hematopoietic disorders. For example, aberrant T-bet expression can be a driving force in inflammatory diseases,3 and several studies have reported augmented IFN-γ production and T-bet expression in CD4+ T cells infiltrating affected lesions in patients with Crohn disease.4,5 T-bet–mediated expression of IFN-γ also appears to play a key role in the pathogenesis of type 1 diabetes, an organ-specific autoimmune disease.6 Notably, a considerable number of aplastic anemia patients show constitutive expression of T-bet, although the mechanism by which this occurs remains unclear.7-9 It is anticipated that understanding the function of T-bet expression in these diseases will be beneficial for the development of new therapeutics.
To that end, the human CD2–T-bet-transgenic (hCD2-T-bet tg) mouse was generated and used to study the contribution made by T-bet to the pathogenesis of inflammatory diseases. T-bet expression in these mice is under the control of the human CD2 promoter, which allows it to be expressed exclusively in CD2-expressing cells.10,11 In the present study, we further explored these mice, focusing in particular on the hematopoietic system, especially hematopoiesis of the mononuclear phagocyte lineage. Unexpectedly, we found that aged transgenic mice homozygous for the hCD2–T-bet tg allele spontaneously developed a pulmonary disease resembling human pulmonary alveolar proteinosis (PAP), a rare lung disorder characterized by the accumulation of surfactant protein within alveolar spaces due to functional defects in alveolar macrophages, and accompanied by local and bone marrow (BM) mononuclear phagocyte dysregulation.
The molecular pathogenesis has been identified as disruption of granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling caused by a genetic mutation of the GM-CSF receptor in most cases of the hereditary form of PAP, and by neutralizing anti–GM-CSF autoantibodies in the idiopathic form of the disease, now referred to as autoimmune PAP.12-18 As compared with the hereditary and autoimmune forms, little is known about the pathogenesis of secondary PAP, which is associated with underlying diseases that include hematologic disorders, immunologic diseases, infections, and various toxic inhalation syndromes. Of note, its association with myelodysplastic syndrome (MDS) has also been well documented.19-23 The findings of the present study demonstrate an unexpected link between T-bet overexpression in T lymphocytes and PAP development caused by functional and maturation impairment of mononuclear phagocytes in the lung, and provide new insight into the molecular pathogenesis of secondary PAP accompanied by hematologic disorders.
Methods
Animals and samples
Generation of the CD2–T-bet transgenic lines has been described previously.11 T-bet transgenic mice were inbred with C57BL/6 mice for at least 8 generations before use in experiments. Genotype was determined by polymerase chain reaction (PCR), using specific primers (see supplemental Table 1 on the Blood Web site). Mice were maintained under specific pathogen-free conditions. The Institutional Animal Care and Use Committee approved all animal experiments; and the Institutional Review Boards of the respective universities, in accordance with the Declaration of Helsinki, approved the animal study and use of human samples.
Microarray analysis
The RNAs from the lungs were extracted using RNeasy Mini Kit (Qiagen, Venlo, The Netherlands). The RNA was reverse transcribed using ReverTra Ace qPCR RT Master Mix (TOYOBO, Osaka, Japan). The prepared complementary RNAs were hybridized with an Agilent microarray slide (Whole Mouse Genome) according to the manufacturer’s instructions (Agilent Technologies, Santa Clara, CA), after which it was scanned with a scanner. The obtained data were analyzed using GeneSpring software (Agilent Technologies).
Colony-forming cell assays
BM cells (BMCs) were seeded at a density of 5 × 104 cells/35-mm dish in methylcellulose medium (MethoCult M3234; STEMCELL Technologies, Vancouver, BC, Canada) supplemented with 50 ng/mL murine stem cell factor (PeproTech, Rocky Hill, NJ), 10 ng/mL IL-3 (PeproTech), 10 ng/mL IL-6 (R&D Systems, Minneapolis, MN), 3 U/mL erythropoietin (PeproTech), and 30 U/mL thrombopoietin (PeproTech). The cells were then cultured at 37°C under 5% CO2. On day 10, the numbers of colonies were counted and stained for colony scoring.
For macrophage-colony stimulating factor (M-CSF) treatment, the prepared BMCs were seeded into MethoCult supplemented with the cytokines mentioned above, plus 10 ng/mL murine M-CSF (PeproTech) and assayed as described above.
Peripheral blood (PB) examinations
Complete blood counts (CBCs) were obtained using an automated counter (CelltacαEK-6358; Nihon Kohden, Tokyo, Japan). For flow cytometric analysis, the pellets of blood cells were stained with a cocktail of diluted directly conjugated fluorescent antibodies (supplemental Table 2) at room temperature for 15 minutes. The cells were then washed with phosphate-buffered saline containing 3% (v/v) fetal bovine serum, centrifuged, and resuspended in the same buffer with 1 µg/mL of propidium iodide.
Immunohistochemistry
After incubation with 3% hydrogen peroxide to inhibit endogenous peroxidase activity, lung sections were immunostained with antisurfactant protein (SP)-A (1:200 dilution, ab115791; Abcam, Cambridge, MA) and anti–T-bet (1:250 dilution, sc-21003; Santa Cruz Biotechnology, Santa Cruz, CA) polyclonal antibodies. The sections were developed using Histofine MAX PO (Nichirei Biosciences, Tokyo, Japan) and diaminobenzidine solution according to the manufacturer’s instructions. Finally, the sections were counterstained with hematoxylin.
Transplantation of splenocytes
For adoptive transfer, the mononuclear cells were obtained from single-cell suspensions from the spleens of T-bettg/tg, T-bettg/wt, and wild-type (WT) mice by using centrifugation with Ficoll-Paque PLUS (GE Healthcare, Little Chalfont, United Kingdom) or the adhesion-depletion method. A million mononuclear cells were then IV injected into CD45.1-Rag2−/− mice (Sankyo Laboratories, Ibaraki, Japan). From the 5th week after transplantation, the mice were subjected to weekly monitoring of their CBCs and flow cytometric analyses to detect T-cell engraftment and subpopulations of myeloid cells. The mice were then analyzed at the 40th week after transplantation.
Flow cytometry
Cells in bronchoalveolar lavage (BAL) fluid, splenocytes, or blood cells were diluted to 2 × 106/100 µL and incubated with a nonspecific binding blocking reagent containing mouse CD16/CD32 (2.4G2) for 15 minutes at 4°C. The collected cells were stained with antibodies for 30 minutes at 4°C and analyzed by a FACSAria II (BD Biosciences, San Jose, CA), after which the data were analyzed using FlowJo software (Tree Star, Ashland, OR). Cell sorting for messenger RNA (mRNA) levels analysis was performed on a FACS Aria II. Some BAL fluid samples were applied to a Mouse Inflammation CBA (BD Biosciences) using FACS Canto II (BD Biosciences) and BD CBA software. Data were analyzed using BD FCAP Array software. Intracellular staining of IFN-γ and T-bet was performed using Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) and splenocytes were stimulated under the presence or absence of 50 ng/mL phorbol 12-myristate 13-acetate, 500 ng/mL ionomycin, and 2 µM monencine for 5 hours. The antibodies used for flow cytometric analysis and the definition of surface antigen expression patterns for hematopoietic stem/progenitor cell populations are listed in supplemental Tables 2 and 3, respectively.
Statistical analysis
Statistical analysis was performed using Microsoft Excel or GraphPad Prism 4 (GraphPad Software, La Jolla, CA). Statistical tests included unpaired or paired Student t tests and ordinary 1-way ANOVA, followed by Tukey’s multiple comparison tests. For nonparametric data sets, the statistical significance was examined using Kruskal-Wallis H tests, followed by Steel-Dwass tests. Survival data were analyzed using the Kaplan-Meier method with the log-rank test. Values of P < .05 were considered significant.
Results
T-bettg/tg mice have reduced survival
To gain insight into the special link between T-bet and disease, we analyzed hCD2–T-bet tg mice.11 We began by monitoring the viability of the littermates from hCD2–T-bettg/wt (T-bettg/wt) mice. Of note, starting at ∼20-weeks of age, more than 80% of the homozygous transgenic (T-bettg/tg) mice became moribund and died under specific pathogen-free conditions, whereas all T-bettg/wt and WT mice survived for the entire observation period of more than 2 years (Figure 1A). To exclude a possibility that the phenotype was the result of gene disruption caused by the transgene integration, we performed a transgene integration analysis using genomic DNA from a T-bettg/wt mouse. The results showed that the transgene was integrated into an intergenic region on chromosome 11, such that no protein-coding and noncoding RNA genes would be disrupted in the T-bettg/tg mice (supplemental Figure 2A). We designed a PCR primer pair to determine the genotype of each mouse using DNA (supplemental Figure 2B).
Aged T-bettg/tg mice spontaneously develop PAP-like disease. (A) Kaplan-Meier plot showing survival among mice with the indicated genotypes (n = 20 in each group). (B) Representative photomicrographs of the lungs of 50-week-old WT, T-bettg/wt, and T-bettg/tg mice. Alveolar spaces in T-bettg/tg mice are filled with acellular eosinophilic material and show inflammatory cell infiltration. H&E staining; bars = 100 µm. (C) Photomicrographs of lung sections from T-bettg/tg mice. The alveolar material is positive for PAS staining and for immunohistochemical staining of mouse SP-A. Bars = 100 µm. (D) Appearance of BAL fluid from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice. (E) Levels of SP-A expression levels in lungs from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice (n = 4 in each group). (F) The heat-map of genes whose expression changes are >10-fold between the genotypes in lung mRNAs. (G) Levels of T-bet mRNA expression levels in lungs from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice (n = 4 in each group).
Aged T-bettg/tg mice spontaneously develop PAP-like disease. (A) Kaplan-Meier plot showing survival among mice with the indicated genotypes (n = 20 in each group). (B) Representative photomicrographs of the lungs of 50-week-old WT, T-bettg/wt, and T-bettg/tg mice. Alveolar spaces in T-bettg/tg mice are filled with acellular eosinophilic material and show inflammatory cell infiltration. H&E staining; bars = 100 µm. (C) Photomicrographs of lung sections from T-bettg/tg mice. The alveolar material is positive for PAS staining and for immunohistochemical staining of mouse SP-A. Bars = 100 µm. (D) Appearance of BAL fluid from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice. (E) Levels of SP-A expression levels in lungs from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice (n = 4 in each group). (F) The heat-map of genes whose expression changes are >10-fold between the genotypes in lung mRNAs. (G) Levels of T-bet mRNA expression levels in lungs from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice (n = 4 in each group).
Aged T-bettg/tg mice spontaneously develop a PAP-like disease
Hematoxylin and eosin (H&E)-stained sections revealed a profound pathology in the lungs and BM of T-bettg/tg mice. In addition to lymphoid hyperplasia around the airways and veins, we observed the presence of acellular eosinophilic material within the alveoli and severe infiltration of the alveolar space by inflammatory cells in the moribund T-bettg/tg mice (Figure 1B). No remarkable pathological findings were observed in other organs, including the spleen, liver, kidney, and small intestine (supplemental Figure 3). Although lymphocyte infiltration was also observed in the lungs of T-bettg/wt mice over 1-year of age, no alveolar accumulation of acellular material was observed. When we further investigated the histology of the lung tissues, we observed that the acellular material that filled the distal air spaces in T-bettg/tg mice stained positively with periodic acid-Schiff (PAS) (Figure 1C). Moreover, immunohistochemical analysis of the lung tissues showed that the material also stained positive for SP-A. Collectively, these findings were indicative of abnormal surfactant accumulation within the alveolar spaces (Figure 1C). Remarkably, BAL fluid collected from T-bettg/tg mice looked considerably more cloudy than that from their T-bettg/wt and WT siblings, which is a characteristic of BAL samples from PAP patients (Figure 1D). Expression analysis with mRNAs from the lungs demonstrated that SP-A expression levels were comparable between the groups (Figure 1E). To verify that this phenotype was not due to off-target effects of the transgene, we evaluated a second transgenic line and found that it also developed a PAP-like phenotype. Taken together, these results strongly suggest that aged T-bettg/tg mice spontaneously develop a pulmonary syndrome resembling human PAP.
Production of proinflammatory cytokines is enhanced in the lungs of PAP mice
To uncover potential mechanisms underlying the observed PAP-like phenotype, we performed a microarray analysis using lung mRNAs from T-bet tg mice. We detected 653 genes whose expression changed by at least 10-fold between genotypes. Consistent with the histologic findings, clustering and gene ontology analyses revealed that genes related to inflammatory and immune responses were enriched in T-bettg/tg lungs (Figure 1F). In addition, quantitative PCR analysis confirmed upregulation of GM-CSF and Th1 genes selectively in T-bettg/tg samples (supplemental Figure 4A). Expression analysis in mice confirmed that the T-bet expression level was approximately 15 and 5 times higher in the lungs of T-bettg/tg and T-bettg/wt mice than in WT mice, respectively (Figure 1G). Although mRNA expression levels of GM-CSF receptors from alveolar macrophages were different between the groups, those of PU.1, a downstream molecule of the GM-CSF signaling, were similar in all 3 groups24 (supplemental Figure 4B-C). We also quantified the inflammatory cytokines in BAL fluids from T-bettg/tg, T-bettg/wt, and WT mice. We found that the concentrations of the proinflammatory cytokines IFN-γ, tumor necrosis factor (TNF), and IL-6, were markedly elevated in the BAL fluid from moribund T-bettg/tg mice, as compared with other genotypes (Figure 2A). T-bettg/tg mice also showed a substantial increase in the concentration of monocyte chemoattractant protein-1 (MCP-1), which is consistent with previous observations made in PAP patients (Figure 2A).25 Although IL-17A was detected in the BAL fluid of WT mice, it was undetectable in the BAL fluid of both T-bettg/wt and T-bettg/tg mice (supplemental Figure 5A). On the other hand, the concentrations of IL-12p70 and IL-10 were similar in all 3 genotypes. Intracellular flow cytometry further demonstrated that T-cell–restricted and transgene-dependent T-bet overexpression among the hematopoietic cells in the spleen without stimulation, and robust IFN-γ overproduction by T cells upon phorbol 12-myristate 13-acetate /ionomycin stimulation, indicated that IFN-γ overproduction in the mice was induced in a T-cell receptor-dependent manner (supplemental Figure 1).
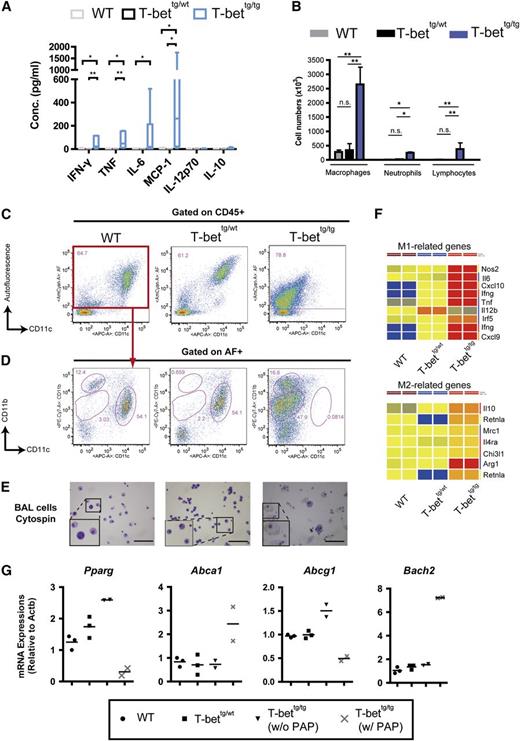
Reorganization of BAL cell populations in T-bettg/tg mice. (A) Concentrations of IFN-γ, TNF, IL-6, MCP-1, IL-12p70, and IL-10 in the BAL fluid from WT, T-bettg/wt, and moribund T-bettg/tg mice at 30- to 50-weeks of age. n = 7, 3, or 5; **P < .01; *P < .05 (Kruskal-Wallis H test, followed by Steel-Dwass tests). (B) Numbers of macrophages, neutrophils, and lymphocytes in BAL fluid from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice. Results are means ± SD; n = 3. **P < .01; *P < .05 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (C-D) Flow cytometry profiles for alveolar macrophages (C) and autofluorescence-positive population (D) among BAL cells gated to the leukocyte population (CD45+ cells). Data are representative of at least 3 mice. (E) Representative cytologies of BAL cells from mice with the indicated genotypes (Hemacolor staining; bars = 50 µm). (F) The heat-maps of M1- and M2-related genes. (G) Levels of Pparg, Abca1, Abcg1, and Bach2 mRNA expression levels in AF+ BAL cells. n = 3, 3, and 4.
Reorganization of BAL cell populations in T-bettg/tg mice. (A) Concentrations of IFN-γ, TNF, IL-6, MCP-1, IL-12p70, and IL-10 in the BAL fluid from WT, T-bettg/wt, and moribund T-bettg/tg mice at 30- to 50-weeks of age. n = 7, 3, or 5; **P < .01; *P < .05 (Kruskal-Wallis H test, followed by Steel-Dwass tests). (B) Numbers of macrophages, neutrophils, and lymphocytes in BAL fluid from 50-week-old WT, T-bettg/wt, and T-bettg/tg mice. Results are means ± SD; n = 3. **P < .01; *P < .05 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (C-D) Flow cytometry profiles for alveolar macrophages (C) and autofluorescence-positive population (D) among BAL cells gated to the leukocyte population (CD45+ cells). Data are representative of at least 3 mice. (E) Representative cytologies of BAL cells from mice with the indicated genotypes (Hemacolor staining; bars = 50 µm). (F) The heat-maps of M1- and M2-related genes. (G) Levels of Pparg, Abca1, Abcg1, and Bach2 mRNA expression levels in AF+ BAL cells. n = 3, 3, and 4.
Subpopulations of BAL cells are altered in the lungs of PAP mice
Because earlier studies had demonstrated that lung inflammation often coexists with a change in the local cellular composition,26,27 we further analyzed the constituents of BAL fluid from mice of each genotype. The numbers of inflammatory cells, including lymphocytes, neutrophils, and most prominently monocytes/macrophages, were significantly higher in the BAL fluid from T-bettg/tg mice than from the other 2 genotypes (Figure 2B). To gain more information on the subpopulation of each leukocyte subset, we also used flow cytometry to analyze the subpopulations of BAL cells. In WT mice, most BAL cells were autofluorescent (AF)+, CD11c+ alveolar macrophages (Figure 2C).28 By contrast, markedly fewer alveolar macrophages were observed in T-bettg/tg lungs (Figure 2C). Instead, we observed an increase in the sizes of the AF+, F4/80low, CD11bhigh, and CD11blow monocyte populations in T-bettg/tg lungs (Figures 2D).26 Although a slight reduction in the alveolar macrophage population was observed in T-bettg/wt mice, most of the AF+ population consisted of CD11c+ alveolar macrophages (Figure 2C). BAL cells from both T-bettg/wt and T-bettg/tg mice showed higher T-cell frequencies than did BAL cells from WT mice (supplemental Figure 5B). Cytological analysis also revealed that among BAL cells from T-bettg/tg mice were large and foamy macrophages, whereas those from T-bettg/wt and WT mice were morphologically normal alveolar macrophages (Figure 2E). Extraction of sets of M1 and M2 macrophage genes from the microarray analysis revealed the upregulation of more M1 genes than M2 genes in T-bettg/tg mice, indicating aberrant activation of both the M1 and M2 programs (Figure 2F). Expression analysis of the genes previously related to lipid handling in macrophages and PAP development29-33 showed a marked reduction of Pparg and Abcg1 mRNA expression levels, but upregulation of Abca1 and Bach2 mRNA expression levels in AF+ populations from mice with PAP phenotype (Figure 2G). In addition, phagocytic assay using fluorescein isothiocyanate-labeled beads demonstrated a reduction of the bead uptake by T-bettg/tg BAL cells compared with WT (supplemental Figure 5C). These findings indicate that changes in leukocyte subpopulations within the alveoli, gene expression levels involved in cholesterol catabolism, and phagocyte function, accompanied by the presence of abundant proinflammatory cytokines, may contribute to the development of PAP in T-bettg/tg mice.
Increased numbers of myeloid cells in the circulation of moribund mice
The massive infiltration of mononuclear phagocytes into the alveoli, led us to hypothesize that PAP development in these mice involved dysregulated mononuclear phagocyte maturation induced by T-bet overexpression in the infiltrated T cells. To test that hypothesis, we performed a series of PB examinations using T-bet tg mice at 25- to 30-weeks of age, which included both moribund and relatively healthy T-bettg/tg mice. The white blood cell (WBC) counts were not significantly altered in either T-bettg/tg or T-bettg/wt mice, but the numbers were higher in T-bettg/tg mice (Figure 3A). Although the differences were not statistically significant, some T-bettg/tg mice showed variable hemoglobin concentrations, hematocrits, and platelet counts (Figure 3B-D). Flow cytometric analyses showed that the numbers of CD11b+ myeloid cells among leukocytes (CD45+) were higher in PB from both T-bettg/tg and T-bettg/wt mice than from WT mice (Figure 3E-F). The numbers of CD11b- cells among leukocytes were comparable in the 3 groups (Figure 3G). Among myeloid populations, we observed higher numbers of Ly6CHigh and Gr-1Mid inflammatory monocytes in PB from T-bettg/tg mice than from either T-bettg/wt or WT mice (Figure 3H). The numbers of neutrophils in PB from T-bettg/tg mice were also higher than the numbers in WT mice but similar to those in T-bettg/wt mice (Figure 3I). Importantly, moribund mice always presented with high numbers of PB inflammatory monocytes and neutrophils, but not high lymphocyte numbers. These results indicate that the development of PAP in T-bettg/tg mice is associated with myeloproliferation.
Number of myeloid cells are increased in the circulation of PAP mice. (A-D) Results of CBCs in PB from WT, T-bettg/wt, and T-bettg/tg mice at 25- to 30-weeks of age: WBCs (A), hemoglobin (B), hematocrit (C), and platelets (D). (E) Representative flow diagrams of living cell-gated populations in the PB of each mouse genotype. Myeloid cells were immunophenotypically defined as CD11b+. The myeloid-gated cells were further analyzed for their levels of Ly6C and Gr-1 expression. Inflammatory monocytes and neutrophils are defined as Gr-1Mid, Ly6CHigh and Gr-1High, and Ly6CMid, respectively. (F-I) Numbers of myeloid cells (F), nonmyeloid cells (CD11b-) (G), inflammatory monocytes (H), and neutrophils (I) in PB from each genotype; n = 3, 5, and 8. *P < .05 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). HCT, hematocrit; HGB, hemoglobin; PLT, platelets.
Number of myeloid cells are increased in the circulation of PAP mice. (A-D) Results of CBCs in PB from WT, T-bettg/wt, and T-bettg/tg mice at 25- to 30-weeks of age: WBCs (A), hemoglobin (B), hematocrit (C), and platelets (D). (E) Representative flow diagrams of living cell-gated populations in the PB of each mouse genotype. Myeloid cells were immunophenotypically defined as CD11b+. The myeloid-gated cells were further analyzed for their levels of Ly6C and Gr-1 expression. Inflammatory monocytes and neutrophils are defined as Gr-1Mid, Ly6CHigh and Gr-1High, and Ly6CMid, respectively. (F-I) Numbers of myeloid cells (F), nonmyeloid cells (CD11b-) (G), inflammatory monocytes (H), and neutrophils (I) in PB from each genotype; n = 3, 5, and 8. *P < .05 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). HCT, hematocrit; HGB, hemoglobin; PLT, platelets.
T-bet tg mice display distinct BM abnormalities
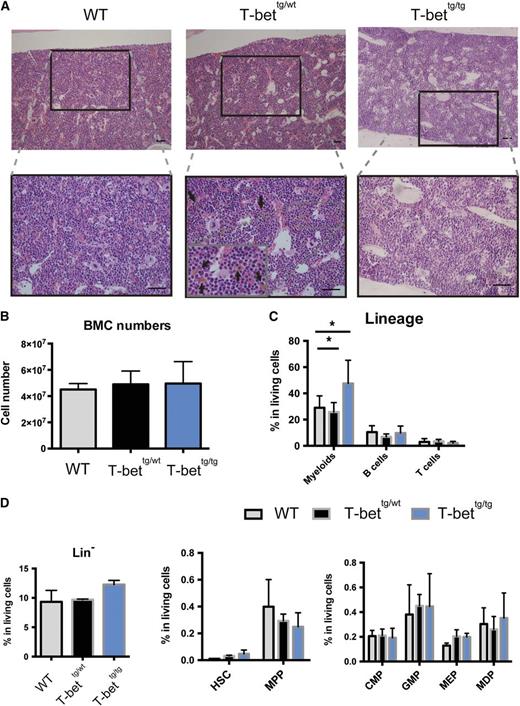
Because secondary PAP is often coupled with various hematologic diseases, we considered the possibility that T-bettg/tg mice might also develop a hematologic disorder. Histopathological analysis of BM from moribund T-bettg/tg mice at 25- to 30-weeks of age showed no signs of BM failure syndromes, but exhibited marked myeloid hyperplasia (Figure 4A). On the contrary, the histopathology of T-bettg/wt BM showed increased phagocytosis of erythroid cells by macrophages (Figure 4A), although the overall numbers of cells in a leg did not significantly differ between the groups (Figure 4B). Among the mature hematopoietic cells in the BM, the frequency of CD11b+ myeloid cells was significantly higher in T-bettg/tg BMCs than T-bettg/wt and WT BMCs, whereas the frequencies of lymphoid cells were comparable between the groups (Figure 4C). Moreover, flow cytometric analysis of hematopoietic stem cell (HSC) and progenitor cell populations showed slightly higher frequencies of HSCs, GM progenitors, and megakaryocyte-erythroid progenitors in both T-bettg/wt and T-bettg/tg BM than in WT BM (Figure 4D). These observations suggest that ectopic T-bet expression in T cells exerted a distinct effect on hematopoiesis in the BM, including augmented erythrophagocytosis in T-bettg/wt BM and myeloid hyperplasia in T-bettg/tg BM.
Distinct BM abnormalities in T-bet tg mice. (A) H&E staining of BM sections (femur) prepared from 30-week-old mice with the indicated genotypes. Arrows indicate yellowish-brown macrophages extensively engulfed erythrocytes. Bar = 100 µm. (B) Total numbers of cells in BM from mice with the indicated genotypes. Results are means ± SD; n = 3, 3, and 6. (C) Percentages of myeloid cells, B cells, and T cells in total living BMCs from 30- to 50-week-old mice with the indicated genotypes. Results are means ± SD; n = 8, 4, and 7. *P < .05 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (D) Percentages of each hematopoietic stem and progenitor compartment in BM from 30- to 50-week-old mice with the indicated genotypes. Results are means ± SD; n = 3 each. CMP, common myeloid progenitors; GMP, granulocyte-macrophage progenitors; MDP, macrophage and dendritic cell progenitors; MEP, megakaryocyte-erythroid progenitors; MMP, multipotent progenitors.
Distinct BM abnormalities in T-bet tg mice. (A) H&E staining of BM sections (femur) prepared from 30-week-old mice with the indicated genotypes. Arrows indicate yellowish-brown macrophages extensively engulfed erythrocytes. Bar = 100 µm. (B) Total numbers of cells in BM from mice with the indicated genotypes. Results are means ± SD; n = 3, 3, and 6. (C) Percentages of myeloid cells, B cells, and T cells in total living BMCs from 30- to 50-week-old mice with the indicated genotypes. Results are means ± SD; n = 8, 4, and 7. *P < .05 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (D) Percentages of each hematopoietic stem and progenitor compartment in BM from 30- to 50-week-old mice with the indicated genotypes. Results are means ± SD; n = 3 each. CMP, common myeloid progenitors; GMP, granulocyte-macrophage progenitors; MDP, macrophage and dendritic cell progenitors; MEP, megakaryocyte-erythroid progenitors; MMP, multipotent progenitors.
BM hematopoietic progenitor cells (HPCs) from T-bettg/tg mice produce augmented dysplastic myeloid cells
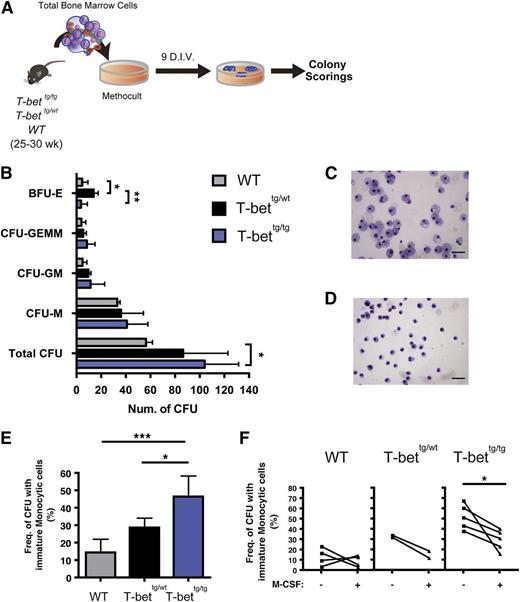
To further explore whether T-bettg/tg mice develop a hematologic disorder, we used colony-forming cell assays to investigate BM HPC function (Figure 5A). We found that BMCs from T-bettg/tg mice generated higher numbers of colony-forming units (CFUs) (Figure 5B, bottom row). Identification of the types of each colony showed that the numbers of each CFU were not significantly elevated, although a trend toward higher CFU numbers was observed with T-bettg/tg BMCs, suggesting that the myeloid differentiation potential remained unchanged (Figure 5B, 2nd to 4th rows). In addition, BMCs from T-bettg/wt mice produced higher numbers of blast forming unit (BFU)-Es than those from T-bettg/tg or WT mice (Figure 5B, top row). Strikingly, a significantly higher number of colonies derived from T-bettg/tg BMCs contained a considerable number of morphologically immature monocytic cells than did colonies from T-bettg/wt or WT BMCs (Figure 5C-E).
BM hematopoietic stem/progenitor cells from T-bettg/tg mice show impaired monocytic differentiation. (A) Experimental protocol for CFC assays. For the input cells, 25- to 30-week-old WT, T-bettg/wt, and T-bettg/tg whole BM cells were used. (B) Results of colony scoring after 9 days of culture in vitro (D.I.V.). Results are means ± SD; n = 5, 4, and 9. *P < .05, **P < .01 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (C-D) Representative cytospin images showing a terminally mature macrophage colony from a culture with WT WBMCs (C), and an immature monocytic colony from T-bettg/tg WBMCs (D). Hemacolor staining. Bar = 50 µm. (E) Frequencies of colonies containing immature monocytic cells. Results are means ± SD; n = 5, 4, and 9. *P < .05; ***P < .001 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (F) Effect of M-CSF addition on the frequencies of colonies containing immature monocytic cells. *P < .05 (paired Student t test). E, erythroid; G, granulocyte; GEMMk, granulocyte, erythoid, macrophage, and megakaryocyte; HSPCs, hematopoietic stem/progenitor cells; M, macrophage, Mk, megakaryocyte.
BM hematopoietic stem/progenitor cells from T-bettg/tg mice show impaired monocytic differentiation. (A) Experimental protocol for CFC assays. For the input cells, 25- to 30-week-old WT, T-bettg/wt, and T-bettg/tg whole BM cells were used. (B) Results of colony scoring after 9 days of culture in vitro (D.I.V.). Results are means ± SD; n = 5, 4, and 9. *P < .05, **P < .01 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (C-D) Representative cytospin images showing a terminally mature macrophage colony from a culture with WT WBMCs (C), and an immature monocytic colony from T-bettg/tg WBMCs (D). Hemacolor staining. Bar = 50 µm. (E) Frequencies of colonies containing immature monocytic cells. Results are means ± SD; n = 5, 4, and 9. *P < .05; ***P < .001 (1-way ANOVA, followed by Tukey’s multiple comparisons tests). (F) Effect of M-CSF addition on the frequencies of colonies containing immature monocytic cells. *P < .05 (paired Student t test). E, erythroid; G, granulocyte; GEMMk, granulocyte, erythoid, macrophage, and megakaryocyte; HSPCs, hematopoietic stem/progenitor cells; M, macrophage, Mk, megakaryocyte.
To determine whether the observed immature cells were monocytic lineage cells, we examined the effects of M-CSF on the dysregulated myeloid differentiation of HPCs from T-bettg/tg mice. The development of monocytic lineage cells descends from HSCs with discrete intermediate progenitor cells, including macrophage and dendritic cell progenitors expressing CD115, the receptor for M-CSF.34 The addition of M-CSF to the culture system resulted in a significant reduction in the numbers of CFUs containing immature monocytic cells (Figure 5F), which implies that the impaired monocytic differentiation in T-bettg/tg can be reversed by the compensatory addition of a lineage-specific cytokine, M-CSF. Thus, BM HPCs from T-bettg/tg mice exhibit monocytic differentiation arrest while producing greater numbers of progenitor cells.
Lymphocytes overexpressing T-bet initiate PAP development and inflammatory monocytosis
We next examined the possibility that the observed phenotypes are due to developmental defects related to ectopic T-bet overexpression. We initially determined whether lymphocytes from T-bettg/tg mice could induce the development of a PAP-like syndrome in lymphocyte-deficient recipients. Specifically, we transplanted CD45.2-expressing lymphocytes from the spleens of moribund T-bettg/tg mice and their controls (T-bettg/wt and WT) into CD45.1-Rag2−/− recipients (Figure 6). H&E- and PAS-stained lung sections from the recipients transplanted with T-bettg/tg splenocytes (tT-bettg/tg) exhibited massive infiltration of hematopoietic cells into the alveolar spaces and accumulation of acellular material there (Figure 6B). By contrast, a slight infiltration of inflammatory cells was seen in recipients transplanted with T-bettg/wt splenocytes (tT-bettg/wt), but PAP did not develop (Figure 6B). Periodic CBCs in the Rag2−/− recipients showed that the WBC counts of tT-bettg/tg mice gradually increased (Figure 6C). In parallel with the increasing WBC counts, flow cytometric PB analysis revealed that the numbers of recipient-driven (CD45.1+) inflammatory monocytes progressively increased in tT-bettg/tg mice, whereas the numbers of recipient-driven neutrophils remained unchanged and were comparable to those in the controls (Figures 6D). Flow cytometric analysis of the donor lymphocytes showed the numbers of T and B cells were comparable between the groups (Figure 6E). These results clearly indicate that the Rag2-deficient recipients developed the PAP-like phenotype, recapitulating the core features of the disease that developed in the primary mice, together with PB inflammatory monocytosis. We further confirmed that T cells isolated from the T-bettg/tg spleen were sufficient to induce PAP-like disease phenotypes in Rag2−/− recipients (supplemental Figure 6A-G). This suggests that, by itself, aberrant overexpression of T-bet in lymphocytes is sufficient to initiate PAP-like disease development in mice.
Overexpression of T-bet in lymphocytes initiates PAP development. (A) Diagram of the experimental design. (B) Representative photomicrographs of the lung sections from Rag2−/− recipients transplanted with splenocytes from WT, T-bettg/wt, or T-bettg/tg mice. H&E (top panels) and PAS (bottom panels) staining. Bars = 100 µm. (C) Changes in WBC counts in PB from Rag2−/− recipients after transplantation of WT or T-bettg/tg splenocytes. (D-E) Changes in the numbers of inflammatory monocytes and neutrophils (D), and of T cells and B cells (E), in the PB of Rag2−/− recipients after transplantation of WT or T-bettg/tg splenocytes. Symbols are means ± SD; n = 4 and 8. ***P < 0.001; **P < .01; *P < .05 (Student t test).
Overexpression of T-bet in lymphocytes initiates PAP development. (A) Diagram of the experimental design. (B) Representative photomicrographs of the lung sections from Rag2−/− recipients transplanted with splenocytes from WT, T-bettg/wt, or T-bettg/tg mice. H&E (top panels) and PAS (bottom panels) staining. Bars = 100 µm. (C) Changes in WBC counts in PB from Rag2−/− recipients after transplantation of WT or T-bettg/tg splenocytes. (D-E) Changes in the numbers of inflammatory monocytes and neutrophils (D), and of T cells and B cells (E), in the PB of Rag2−/− recipients after transplantation of WT or T-bettg/tg splenocytes. Symbols are means ± SD; n = 4 and 8. ***P < 0.001; **P < .01; *P < .05 (Student t test).
T-bet is overexpressed in the lungs of secondary PAP patients
The results obtained with T-bettg/tg mice indicate that a PAP-like phenotype occurs secondary to T-bet overexpression in T cells, accompanied by enhanced production of immature monocytes in the BM. To evaluate the physiological relevance of these findings, we investigated a clinical sample from a secondary PAP patient with MDS (RAEB2, trisomy 8). H&E staining of the specimens showed augmented lymphocyte infiltration in secondary PAP, as compared with autoimmune PAP (supplemental Figure 7A). Immunohistochemical analysis of the specimen revealed infiltration of the lungs by T-bet–positive lymphocytes in secondary PAP patients and T-bettg/tg sections, but not in normal and autoimmune PAP lungs (supplemental Figure 7B-C). Similarly, we found that T-bet expression in a secondary PAP specimen was ∼10× higher than in an autoimmune PAP specimen (supplemental Figure 7D). These results suggest that T-bet overexpression in T lymphocytes may have a role in the pathogenesis of secondary PAP related to hematopoietic disorders.
Discussion
The findings in the present study demonstrate that the level of T-bet expression in T cells critically influences mononuclear phagocyte function. Taking the findings in earlier studies and the transgene analysis into account, the phenotypic difference between T-bettg/wt and T-bettg/tg likely reflects the difference in T-bet expression levels in T cells.35,36 Moreover, we were able to rule out the possible disruption of gene regulatory sequences due to the transgene homozygosity, as PAP development was observed in the other transgenic lines.
All of the features displayed in the moribund T-bettg/tg mice represent the main clinical and laboratory features of PAP. Subsequent in vivo studies indicated that our transgenic mice developed PAP-like disease secondary to aberrant T-bet overexpression in T cells, but not primarily through a defect intrinsic to alveolar macrophages. To date, there have been a few studies reporting the development of secondary PAP disease models.37 One showed the development of secondary PAP in scid/scid mice, presumably recapitulating secondary PAP patients with immunodeficiency, although the mechanism has not yet been identified.38 In the present model, it is clearly indicated that secondary PAP developed under aberrant T-cell activation by T-bet with monocytosis. On the basis of this finding, we suggest that T-bet–overexpressing T cells act to initiate the pathogenesis of secondary PAP.
Alveolar macrophages not only play a central role in pulmonary lipid homeostasis, but they also represent the first line of defense in the alveoli against invading pathogens and inhaled microbes, and their absence results in reduced clearance during infections.39,40 This functional dichotomy is achieved through polarization of alveolar macrophages with distinct functional phenotypes, depending upon different stimuli, and alteration of their behavior is observed under various pathological conditions.26,41,42 For example, alveolar macrophages differentiate into classically activated M1 macrophages in the presence of IFN-γ and other stimuli, including lipopolysaccharide, and exhibit greater microbicidal activity with enhanced nitric oxide synthase, TNF, and IL-6 production.43,44 Flow cytometric and cytogenetic analyses clearly indicated that reorganized macrophages, in addition to T-cell infiltration of the T-bettg/tg alveoli were mainly M1 macrophages, which were different from T-bettg/wt and WT alveolar macrophages. Moreover, we observed marked increases in the levels of the proinflammatory cytokines IFN-γ, TNF, and IL-6 in the lungs of T-bettg/tg mice. Expression analysis with BAL cells demonstrated a marked reduction of Pparg and Abcg1 expression levels, both key regulators of lipid metabolism in macrophages where its deficiencies result in foamy macrophage generation and PAP development. Studies have also demonstrated critical roles of PPARγ in lung macrophages in regulating the resolution phase of inflammation and integrity of its functions.45,46 These findings suggest that those phenotypical alterations in macrophages triggered by the T cells in T-bettg/tg alveoli, lead to loss of the cells responsible for lipid metabolism.
We were able to provide additional evidence that expanded hematopoiesis of myeloid cells is involved in the progression of the PAP-like disease. In particular, we found an association between the development of PAP and a progressive increase in circulating inflammatory monocytes, an immature progenitor cell of infiltrating macrophages. The actions of inflammatory monocytes are detrimental in acute lung injury and contribute to infection-related mortality.47 We also observed a considerable increase in the levels of MCP-1 in the lungs of PAP mice. Several lines of evidence suggest that CCR2/MCP-1 interactions significantly contribute to monocyte, macrophage, and T-cell recruitment to sites of inflammation.48-50 This suggests the infiltration of circulating inflammatory monocytes into the lungs of PAP mice may be mediated through an interaction between MCP-1 and its receptor, CCR2. Identifying the mechanisms by which MCP-1 production is selectively augmented in the lung is intriguing, but will require further investigation. Collectively, these findings suggest that PAP development in T-bettg/tg mice may require infiltration of the alveoli by both T-bet–overexpressing T cells and myeloid cells.
We demonstrated both in vitro and in vivo that there is an acquired dysregulation of myeloid differentiation in T-bettg/tg mice. These findings are in part consistent with recent reports on microbial infections and chronic inflammatory diseases, which found that HSC/HPC cells were directly stimulated by environmental cues, including proinflammatory cytokines.51,52 For example, Griseri et al recently showed that inflammatory cytokines mediate dysregulated hematopoiesis at the HSC level in IL-23–dependent colitis.53 In addition, a relationship between T-bet overexpression and BM failure has been demonstrated in patients with aplastic anemia and MDS.9,54-58 It is worthwhile mentioning that Sca-1 becomes aberrantly expressed and some of the HSCs in this case are in fact myeloid progenitors, when IFNs are upregulated as in the current transgenic mouse.59,60 Taking these previous reports into account, there could have been an increase in the frequency of myeloid progenitors in the BM of T-bet tg mice, which is also in line with the result of the CFC assay. Notably, we found that monocytic differentiation became nonintrinsically impaired at the HPC level in T-bettg/tg BM. These findings, together with the substantial PB monocytosis and their augmented pulmonary infiltration, suggests that some maturation programs within monocytes or more immature progenitor cells toward macrophages are dysregulated by T-bet overexpression, leading to the reduction of the mature alveolar macrophage population.
The current study identified acquired downregulation of genes known to induce PAP development, but the mechanism by which those gene expressions were altered in the mononuclear phagocyte lineage is unclear. Although the GM-CSF–PU.1 axis has been shown to play a pivotal role in PAP pathogenesis,61 expression analyses revealed that T-bettg/tg mice do not develop PAP due to downregulation of PU.1 in lung macrophages. Nonetheless, a study has demonstrated that PPARγ overexpression in GM-CSF–knockout lung macrophages, which are deficient in PPARγ expression, rescues alveolar surfactant accumulation, indicating potential regulation of PPARγ expression by GM-CSF signaling.62 It is, therefore, possible that acquired downregulation of other GM-CSF signaling pathways may be involved in the pathogenesis. The association between increased IFN-γ and reduced PPARγ has been described.45 Investigating the mice in IFN-γ- and GM-CSF–deficient backgrounds may address the question. Even though further investigation is clearly in order, studies with human specimens suggested that T-bet overexpression and increased pulmonary infiltration of lymphocytes may be involved in the pathogenesis of secondary PAP, particularly PAP with aberrant hematopoiesis of myeloid cells. From these observations, it appears the present model may recapitulate some aspects of the disease progression seen in secondary PAP patients with BM failure syndrome. Taken together, the findings of the present study provide new insight into the potential pathogenesis of PAP accompanied by hematologic disorders.
The microarray data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE62637).
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Takeo Endo and Hidetoshi Yanai for their clinical advice, Dr Masataka Kasai for his critical review of the manuscript, and Dr Tsuyoshi Ito for technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas (S.K.), Scientific Research-C (Y.I.), and Exploratory Research (Y.M.) from the Japan Society for the Promotion of Science.
Authorship
Contribution: S.I. and N.K. performed the analysis, drafted the manuscript, and designed the study; E.N. performed the transgene integration site analysis; Y. M. and M.M. contributed to acquisition and analysis of the flow cytometry and the PCR data; K.Y. and S.T. generated and maintained transgenic mice; H.N. revised the manuscript; and Y.I. and S.K. were responsible for the overall design of the study and revision of the manuscript.
Conflict-of-interest disclosure: H.N. is a founder, shareholder, and a scientific adviser of iCELL Inc., ReproCELL Inc., and Megakaryon Inc. The remaining authors declare no competing financial interests.
Correspondence: Shin Kaneko, Shin Kaneko Laboratory, Center for iPS Cell Research and Application, Kyoto University, 53 Kawaharacho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: kaneko.shin@cira.kyoto-u.ac.jp; and Yukio Ishii, Department of Respiratory Medicine, University of Tsukuba, 1-1-1 Tennoudai, Tsukuba, Ibaraki 305-8575, Japan; e-mail: ishii-y@md.tsukuba.ac.jp.
References
Author notes
S.I. and N.K. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal