In this issue of Blood, Chen et al1 and Kameda et al2 demonstrate that Tet2 loss has 2 effects in Jak2V617F mice: it increases both the severity of the myeloproliferative disorders and the self-renewal properties of the Jak2V617F hematopoietic stem cells (HSCs).
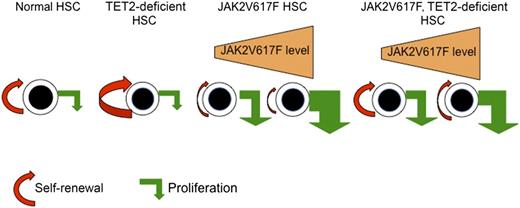
Model for the regulation of HSC self-renewal and proliferation by TET2 loss, JAK2V617F, and the combination of JAK2V617F and TET2 loss. TET2 loss increases the self-renewal of HSCs without significant effects on their proliferation, explaining that TET2-deficient HSCs outcompete normal HSCs and lead to clonal hematopoiesis. The effects of JAK2V617F on HSCs are dependent on the level of expression and yet-unknown parameters. At low levels of expression, JAK2V617F induces the proliferation of HSCs but slightly decreases self-renewal. The proliferation effect being predominant, JAK2V617F HSCs are capable of outcompeting wild-type HSCs. At high levels of expression, JAK2V617F may more profoundly alter the functions of HSCs. The TET2 loss restores the function of JAK2V617F by increasing self-renewal. The restoration is complete or incomplete depending on the defect of the JAK2V617F HSCs. Overall, TET2 loss increases the fitness of JAK2V617F to induce an MPN.
Model for the regulation of HSC self-renewal and proliferation by TET2 loss, JAK2V617F, and the combination of JAK2V617F and TET2 loss. TET2 loss increases the self-renewal of HSCs without significant effects on their proliferation, explaining that TET2-deficient HSCs outcompete normal HSCs and lead to clonal hematopoiesis. The effects of JAK2V617F on HSCs are dependent on the level of expression and yet-unknown parameters. At low levels of expression, JAK2V617F induces the proliferation of HSCs but slightly decreases self-renewal. The proliferation effect being predominant, JAK2V617F HSCs are capable of outcompeting wild-type HSCs. At high levels of expression, JAK2V617F may more profoundly alter the functions of HSCs. The TET2 loss restores the function of JAK2V617F by increasing self-renewal. The restoration is complete or incomplete depending on the defect of the JAK2V617F HSCs. Overall, TET2 loss increases the fitness of JAK2V617F to induce an MPN.
BCR-ABL-negative classical myeloproliferative neoplasms (MPNs) include 3 disorders—essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis—which are driven by JAK2 activation. JAK2V617F, a gain-of-function mutation, is the most frequent genetic alteration. It is present in more than 70% of MPNs and leads to a cytokine hypersensitivity.3 JAK2V617F is really the driver of the disease because it induces a myeloproliferative disorder in all tested murine models, sometimes with extremely different phenotypes that can range from a benign thrombocytosis to a lethal myelofibrosis.3 This range is well illustrated in the 2 present studies: the model used by Chen et al is an inducible Jak2V617F knockin, which induces a polycythemialike disorder1 ; that of Kameda et al is a constitutive transgenic Jak2V617F mouse model that leads to a lethal myelofibrosis after transplantation.2 The reasons for these differences among models are presently unclear, but the level of JAK2V617F expression is the principal determinant in the phenotype, even if the origin (human or murine) of JAK2V617F may also play a role.4 In addition, it is possible that other factors that change JAK2 expression during differentiation may modify the phenotype of the disorder.
Thus, in many ways, JAK2V617F has similarities with BCR-ABL and it could be expected that it is the unique genetic somatic alteration that drives MPNs in humans. This remains a major debate. Indeed, during these last years, it has been found that several mutations are associated with JAK2V617F that could either predate JAK2V617F or appear as secondary events.3 However, it remains that in a majority of patients with ET and PV, JAK2V617F is the unique somatic driver.
Why should other mutations be necessary? The HSC is the Achilles’ heel of the JAK2V617F disease. In humans, the dominance of the JAK2V617F clone in most PV and ET takes place in late stages of differentiation. In murine models, Jak2V617F changes the properties of HSCs by inducing their proliferation, a property usually associated with a loss of self-renewal capacity.5 Depending on the murine model, this ability can have minor consequences—Jak2V617F HSCs were able to outcompete wild-type HSCs in the first transplantation as seen in the model used by Chen et al1 —or it may induce drastic consequences, leading to completely defective HSCs as seen in the model used by Kameda et al.2 However, it is confirmed that a single Jak2V617F HSC, when Jak2V617F is appropriately expressed, can induce with low efficiency6 an MPN in the mouse, suggesting that JAK2V617F is a unique genetic element capable of giving rise to an MPN but with low penetrance and long latency.
Among the associated mutations, some are important to promote clonal hematopoiesis and to increase the fitness of the disease. TET2 mutations found in about 15% of MPNs are the paradigm of this class of mutations and have been initially identified in a subgroup of PV patients with a clonal dominance in the early phase of hematopoiesis.7 In mice, Tet2 deficiency increases the self-renewal capacities of HSCs.8 Furthermore, during aging, hematopoiesis becomes clonal in approximately 40% of healthy women when studied by skewing of the X-chromosome inactivation patterns. In about 6% of those cases, mutations in the TET2 gene are found even in the absence of any hematologic disorder, demonstrating that TET2 mutations are capable of giving rise to a clonal hematopoiesis, possibly favoring secondarily the occurrence of the JAK2V617F mutation.9
In the 2 present studies, the authors directly demonstrate that TET2 loss is capable of completely1 or partially2 restoring the decreased self-renewal capacities of JAK2V617F HSCs by facilitating secondary transplantations. The effect of TET2 on JAK2V617F HSCs can be extended to other mutated HSCs, as previously demonstrated for ASXL1,8 which may explain the presence of TET2 mutations in a large number of hematologic malignancies.
However, the effects of Tet2 loss were not restricted to HSCs but also led to a more severe MPN in mice and accelerated death compared to the single Jak2V617F mutation in the absence of leukemia occurrence. One of the most striking differences is the increased extramedullary hematopoiesis as revealed by a massive splenomegaly in the double-mutant mice. Why Tet2 loss induces an increase in extramedullary hematopoiesis is unknown. It may be the consequence of increased myeloproliferation, thus mimicking a stress hematopoiesis, or changes in HSC trafficking and homing properties. In humans, there is presently no evidence of worse prognosis when TET2 predates JAK2V617F, in contrast to secondary occurrence of TET2 mutations that seem to be associated with MPN progression.
In the 2 studies, the authors have tried to tackle the mechanisms by which TET2 increased the self-renewal capacities of JAK2V617F properties by using a gene-profiling approach. Overall changes were subtle and Tet2 loss led mainly to an increased self-renewal signature.1 In the model of Kameda et al,2 Jak2V617F decreased this self-renewal signature, and the combined loss of Tet2 only partially restored it, in agreement with what they observed in their mouse model.
The precise mechanism by which TET2 loss increases self-renewal capacities of HSCs is still poorly known. However, because TET2 mutations are associated with aging in humans,9 it cannot be excluded that TET2 loss may have a more striking effect on older rather than younger HSCs.
These 2 studies may be important for understanding the effects of therapy. Interferon-α is presently the only drug capable of inducing a molecular remission in PV, and there is evidence that it is through exhaustion of JAK2V617F HSCs. Thus, it is possible that TET2 loss may impair this therapeutic effect, more particularly if it predates JAK2V617F.10 Further studies using such mouse models may be important to determine the interest and the limit of interferon therapy in MPNs.
Conflict-of-interest disclosure: The authors declare no competing financial interests.