Key Points
MPA suppresses ribosomal RNA (rRNA) synthesis and cell proliferation in T cells through TIF-IA, a GTP binding protein.
The combination of MPA and sotrastaurin potently suppresses T-cell proliferation and inhibits IL-2 secretion through TIF-IA and ErbB3-binding protein 1 (Ebp1).
Abstract
Mycophenolic acid (MPA) is the active metabolite of mycophenolate mofetil, an effective immunosuppressive drug. Both MPA and mycophenolate mofetil are highly specific inhibitors of guanine nucleotide synthesis and of T-cell activation. However, the mechanism by which guanine nucleotide depletion suppresses T-cell activation is unknown. Depletion of GTP inhibits ribosomal RNA synthesis in T cells by inhibiting transcription initiation factor I (TIF-IA), a GTP-binding protein that recruits RNA polymerase I to the ribosomal DNA promoter. TIF-IA–GTP binds the ErbB3-binding protein 1, and together they enhance the transcription of proliferating cell nuclear antigen (PCNA). GTP binding by TIF-IA and ErbB3-binding protein 1 phosphorylation by protein kinase C δ are both required for optimal PCNA expression. The protein kinase C inhibitor sotrastaurin markedly potentiates the inhibition of ribosomal RNA synthesis, PCNA expression, and T-cell activation induced by MPA, suggesting that the combination of the two agents are more highly effective than either alone in inducing immunosuppression.
Introduction
The inhibition of T-cell activation is essential in the treatment of certain autoimmune diseases and in the prevention of graft-versus-host disease that accompanies hematopoietic stem cell transplantation. Mycophenolate mofetil (MMF/Cellcept) has been used in combination with other immunosuppressive drugs to treat graft-versus-host disease, and is a potent, selective, and reversible inhibitor of the type II isoform of inosine monophosphate dehydrogenase, an enzyme involved in the de novo biosynthesis of guanine nucleotides.1,2 Mycophenolic acid (MPA), the active ingredient in MMF, depletes guanine nucleotides in T and B lymphocytes, resulting in the inhibition of lymphocyte proliferation and suppression of cell-mediated immune responses and antibody production.2,3 The depletion of guanine nucleotides by MPA has also been shown by ourselves and others to inhibit the synthesis of ribosomal RNA (rRNA),4,5 although the mechanism underlying this effect has not been identified.
Transcription initiation factor I (TIF-IA), a key intermediate in the overall regulation of rRNA synthesis,6,7 is ubiquitously expressed in mammalian cells8-10 and is required to recruit Pol I to the ribosomal DNA (rDNA) promoter to generate a productive transcription initiation complex.9-11 TIF-IA is phosphorylated at multiple sites by a number of protein kinases12-14 and its posttranslational modifications constitute one of the most important mechanisms by which growth signaling pathways regulate rRNA synthesis. The ErbB3-binding protein 1 (Ebp1) is also ubiquitously expressed in human tissues15 and is highly conserved throughout evolution.16 A number of studies have indicated that Ebp1 plays varied and important roles in the regulation of cell proliferation and differentiation.17-21 Ebp1 encodes two alternatively spliced isoforms, p48 and p42.22 The predominant p48 isoform can promote cell proliferation and survival, in part through enhancing polyubiquitination and degradation of the tumor suppressor p53 through the E3 ligase HDM2,23,24 whereas the p42 isoform has been regarded as a tumor suppressor.25,26 In addition to ErbB3, the long form of Ebp1 interacts with a variety of other proteins relevant to cell proliferation, including nucleophosmin and Akt.27,28 A specific role for Ebp1 as a regulator of rRNA synthesis has not been established, although it has been postulated to interfere with rRNA processing and ribosome biogenesis when localized in the nucleolus.17
After initially noting that the TIF-IA sequence contained a consensus binding site for GTP, we asked (1) whether the binding of GTP was required for TIF-IA function in regulating rRNA synthesis in T lymphocytes, and if so, (2) whether the binding of GTP resulted in additional protein-protein interactions of TIF-IA. The results of these studies demonstrate that GTP is required for the interaction of TIF-IA with Ebp1, and that both are important contributors to the regulation of rRNA synthesis and to T-lymphocyte activation. These data provide both a further explanation of the mechanism-of-action of MPA and an additional target that might be exploited to enhance its immunosuppressive activity.
Methods
Human patient samples, and primary T-cell isolation and culture
After informed consent under Stanford University’s Institutional Review Board protocol number 14734, peripheral blood mononuclear cells (PBMCs) were obtained from patients with systemic lupus erythematosus through the Stanford Immunologic and Rheumatic Diseases Registry and Biospecimen Repository. Viable PBMCs were isolated using Ficoll-Hypaque separation and cryopreserved until use. All patients were on MMF at a stable dose for more than 3 months at the time blood was obtained.
T cells were isolated from PBMCs and purified using a T-cell Isolation Kit (Stemcell Technologies, Vancouver, Canada). T-cell populations were 87.5 ± 6.0 pure, as determined by flow cytometry using anti-CD3 antibody. Cell pellets were viably frozen in RPMI medium supplemented with 2% human AB serum (Cellgro) and 10% dimethylsulfoxide (DMSO) (Sigma-Aldrich) at −80°C until use.
For longer term cultures, PBMCs were cultured in 96-well round-bottom plates precoated with 1 μg/mL anti-CD3 (OKT3, BioLegend) and 1 μg/mL anti-CD28 (CD28.2, BioLegend) in RPMI medium supplemented with 2% human AB serum (Cellgro), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were first cultured for 5 days to generate CD3+ T cells. MPA was added at that time point, referred to as “day 0” of the experiment.
Synthetic small interfering RNA (siRNA) oligonucleotides
The siGENOME SMARTpool for siRNAs was purchased from Thermo Scientific (Lafayette, CO). Scrambled control RNA (SCR) was used as a control. The target sequences for siRNAs are shown in supplemental Table 1 on the Blood Web site. In each instance, protein expression was verified as being >50% of the control value.
Chromatin immunoprecipitation (ChIP) assays
ChIP was performed as described by the manufacturer (Pierce, Rockford, IL). Precleared chromatin was incubated overnight by rotation with 4 μg of Pol I antibody or IgG antibody as a negative control. Immunoprecipitates were resuspended in 50 μL Tris-EDTA buffer. Inputs and immunoprecipitated DNA samples were quantified by quantitative polymerase chain reaction on a 7900T Fast Real-Time PCR System (Applied Biosystems, Foster, CA).
RNA labeling and analysis
Cells were washed and incubated in phosphate-free Dulbecco’s modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum for 2 hours, followed by 1 hour labeling with 0.5 mCi [32P] orthophosphate (PerkinElmer). Total RNA was extracted with TRIzol (Life Technologies) following the manufacturer’s protocol. Equal amounts of RNA (10 μg) were separated on a 1.2% MOPS formaldehyde gel. The gel was dried and visualized by autoradiography.
Interleukin (IL-2) measurements
Measurement of IL-2 production in T cells was performed with the enzyme-linked immunosorbent assay kit from BioLegend (San Diego, CA) following the manufacturer’s instructions. CD3+ T cells were seeded in IL-2 antibody-coated 96-well plates. A biotin-conjugated anti-human IL-2 detection antibody and avidin-conjugated horseradish peroxidase were added and absorbance was read at 450 nm.
Statistical analysis
Where indicated, results were compared using the unpaired Student t test with values obtained from at least 3 independent experiments. P < .05 was considered significant.
Results
MPA inhibits rRNA synthesis and cell proliferation in T cells through TIF-IA
We employed both cultured primary human T cells and a Jurkat T-cell line to elucidate the relationship between MPA and rRNA synthesis. MPA suppresses rRNA synthesis in primary T cells as measured by the level of 5′ external transcribed spacer (ETS) pre-rRNA and Pol I recruitment to rDNA (Figure 1A, left and middle). Cell proliferation as determined by colorimetric MTS assay and carboxyfluorescein diacetate succinimidyl ester assays is also reduced (Figure 1A, right and supplemental Figure 1C). MPA also inhibits the expression of proliferating cell nuclear antigen (PCNA) protein29-31 , with a corresponding increase in p-H2AX and p53 levels (Figure 1A, right). Similar effects of MPA on rRNA synthesis, PCNA, p-H2AX, and p53 levels and cell proliferation were observed in Jurkat T cells (supplemental Figure 1A). To further validate these observations, T lymphocytes were obtained from individuals on daily MPA treatment of autoimmune diseases and from untreated controls. T cells derived from individuals on MPA had reduced levels of rRNA synthesis, PCNA protein expression, and cell proliferation as compared with control samples (Figure 1B and supplemental Figure 1B).
Inhibition of rRNA synthesis and cell proliferation in T cells by MPA. (A) Effects of MPA on rRNA synthesis and cell proliferation in cultured primary T cells. Primary T cells were isolated from PBMCs of healthy donor blood and cultured in stimulation medium (see “Methods”). (Left and middle) Cells were treated with DMSO or MPA (100 nM) for 3 hours. RNA was extracted for measurement of 5′ETS pre-rRNA and RNA was labeled with [32P] (left). ChIP assay was performed to measure the level of Pol I recruited to rDNA promoter (middle). GAPDH (left) or 10% input (middle) were used as internal controls and samples were run in triplicate. Cells were cultured in proliferation medium in the presence of DMSO or MPA (100 nM) for 24 hours. MTS assay and western blot were performed as shown (right). (B) Effects of MPA on rRNA synthesis and cell proliferation in primary T cells from patients treated with MPA. T cells were isolated from PBMCs of healthy donors (n = 6) or individuals treated with MPA (n = 5). RNA was extracted for the measurement of 5′ETS pre-rRNA (left), ChIP assay was performed to measure the level of Pol I recruited to rDNA promoter (middle), and MTS assay and western blot were performed as shown (right). Significance was determined using the Student t test. Individual values are shown in supplemental Figure 1B. CON, control; EtBr, ethidium bromide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IGS, intergenic spacer; OD, optical density.
Inhibition of rRNA synthesis and cell proliferation in T cells by MPA. (A) Effects of MPA on rRNA synthesis and cell proliferation in cultured primary T cells. Primary T cells were isolated from PBMCs of healthy donor blood and cultured in stimulation medium (see “Methods”). (Left and middle) Cells were treated with DMSO or MPA (100 nM) for 3 hours. RNA was extracted for measurement of 5′ETS pre-rRNA and RNA was labeled with [32P] (left). ChIP assay was performed to measure the level of Pol I recruited to rDNA promoter (middle). GAPDH (left) or 10% input (middle) were used as internal controls and samples were run in triplicate. Cells were cultured in proliferation medium in the presence of DMSO or MPA (100 nM) for 24 hours. MTS assay and western blot were performed as shown (right). (B) Effects of MPA on rRNA synthesis and cell proliferation in primary T cells from patients treated with MPA. T cells were isolated from PBMCs of healthy donors (n = 6) or individuals treated with MPA (n = 5). RNA was extracted for the measurement of 5′ETS pre-rRNA (left), ChIP assay was performed to measure the level of Pol I recruited to rDNA promoter (middle), and MTS assay and western blot were performed as shown (right). Significance was determined using the Student t test. Individual values are shown in supplemental Figure 1B. CON, control; EtBr, ethidium bromide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IGS, intergenic spacer; OD, optical density.
Stimulation of cultured T lymphocytes using anti-CD3 and anti-CD28 antibodies resulted in increased expression of TIF-IA (Figure 2A), whereas a reduction in TIF-IA expression by siRNA decreased both rRNA synthesis and cell proliferation (Figure 2B and supplemental Figure 2A,F). MPA treatment did not affect the total level of TIF-IA but induced the translocation to the periphery of nucleolus, a site where rRNA transcription is inactive32 (supplemental Figure 2B). It also resulted in the dissociation of TIF-IA from Pol I in T cells (Figure 2C, left), as well as a decrease in Pol I binding to the rDNA promoter and a decrease in rRNA synthesis at 1 to 2 hours (Figure 2C, middle and right). A similar decrease in the association of TIF-IA with Pol I occurred in T cells isolated from individuals treated with MPA (Figure 2D). Of additional interest, MPA treatment of short time intervals of 10 to 30 minutes increased both the interaction of TIF-IA with Pol I and rRNA synthesis (Figure 2C). The addition of guanosine, which has been shown to replete guanine nucleotide pools after inosine monophosphate dehydrogenase inhibition,33,34 to cells pretreated with MPA, reversed these effects of MPA (supplemental Figure 2C-E). Depletion of TIF-IA enhanced the effects of MPA in inhibiting both rRNA synthesis and cell proliferation (Figure 2E). These data indicate that GTP depletion resulting from MPA treatment alters TIF-IA localization and function, findings that would explain the inhibition of RNA synthesis and cell proliferation in T cells.
Effects of MPA treatment on TIF-IA–regulated rRNA synthesis in T cells. (A) Expression of TIF-IA, PCNA, and Ebp1 protein in T cells stimulated for 5 days with anti-CD3 and anti-CD28. Primary T cells were isolated from PBMCs and cultured in control medium or medium containing anti-CD3/CD28. (B) Effects of TIF-IA depletion on rRNA synthesis and proliferation in primary T cells. Cells were cultured in proliferation medium and then transfected with SCR or siTIF-IA (20 nM) for 36 hours. RNA was extracted for measurement of 5′ETS pre-rRNA (left), Pol I recruitment to the rDNA promoter (middle), and MTS assay and western blot were performed as shown (right). (C) Effects of MPA on TIF-IA regulation of rRNA synthesis. Primary T cells were cultured in proliferation medium and treated with MPA for the indicated times. TIF-IA was immunoprecipitated from cell lysate and blots were probed with anti-Pol I antibody (left), ChIP assay with Pol I antibody (middle), and 5′ETS pre-rRNA levels and RNA labeling with [32P] (right). (D) Effects of MPA on TIF-IA binding with Pol I in cells from individuals treated with MPA. TIF-IA was immunoprecipitated from cell lysate that was pooled from healthy donors (n = 6) or individuals treated with MPA (n = 5), and blots were probed with anti-Pol I antibody. (E) Effects of TIF-IA depletion on rRNA synthesis and cell proliferation in T cells treated with MPA. The cells were transfected with SCR or siTIF-IA (20 nM) for 24 hours, then treated with MPA (100 nM) for 3 hours (left and middle) or 24 hours (right). 5′ETS pre-rRNA (left), ChIP assay (middle), and MTS and western blot (right).
Effects of MPA treatment on TIF-IA–regulated rRNA synthesis in T cells. (A) Expression of TIF-IA, PCNA, and Ebp1 protein in T cells stimulated for 5 days with anti-CD3 and anti-CD28. Primary T cells were isolated from PBMCs and cultured in control medium or medium containing anti-CD3/CD28. (B) Effects of TIF-IA depletion on rRNA synthesis and proliferation in primary T cells. Cells were cultured in proliferation medium and then transfected with SCR or siTIF-IA (20 nM) for 36 hours. RNA was extracted for measurement of 5′ETS pre-rRNA (left), Pol I recruitment to the rDNA promoter (middle), and MTS assay and western blot were performed as shown (right). (C) Effects of MPA on TIF-IA regulation of rRNA synthesis. Primary T cells were cultured in proliferation medium and treated with MPA for the indicated times. TIF-IA was immunoprecipitated from cell lysate and blots were probed with anti-Pol I antibody (left), ChIP assay with Pol I antibody (middle), and 5′ETS pre-rRNA levels and RNA labeling with [32P] (right). (D) Effects of MPA on TIF-IA binding with Pol I in cells from individuals treated with MPA. TIF-IA was immunoprecipitated from cell lysate that was pooled from healthy donors (n = 6) or individuals treated with MPA (n = 5), and blots were probed with anti-Pol I antibody. (E) Effects of TIF-IA depletion on rRNA synthesis and cell proliferation in T cells treated with MPA. The cells were transfected with SCR or siTIF-IA (20 nM) for 24 hours, then treated with MPA (100 nM) for 3 hours (left and middle) or 24 hours (right). 5′ETS pre-rRNA (left), ChIP assay (middle), and MTS and western blot (right).
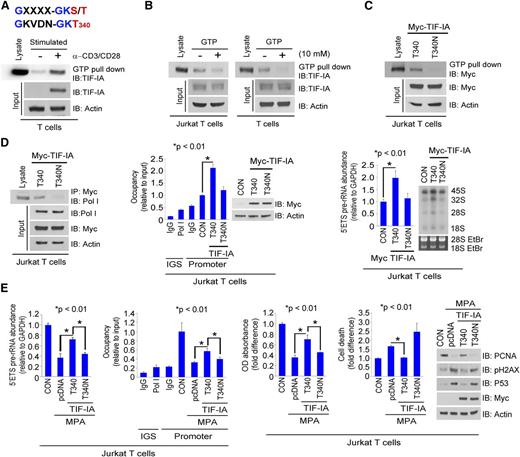
GTP binding is required for TIF-IA regulation of rRNA synthesis
The TIF-IA protein contains the consensus GTP binding motif GXXXX-GKS/T340 (Figure 3A, top). To determine whether TIF-IA binds GTP, lysates from primary T cells and 293T cells were incubated with GTP immobilized on sepharose beads. As shown in Figure 3A, TIF-IA bound to the beads in the absence but not in the presence of excess GTP (Figure 3B and supplemental Figure 3A). Similar results were obtained with recombinant Myc–TIF-IA and GST–TIF-IA proteins when each was expressed in 293T cells (supplemental Figure 3B). Mutation of TIF-IA at the binding site (T340N) inhibited its interaction with GTP-sepharose (Figure 3C and supplemental Figure 3C). We conclude from these data that TIF-IA is a GTP binding protein. As shown in Figure 3D and supplemental Figure 3D, mutation at the T340N site also reduced the interaction of TIF-IA with Pol I and reduced Pol I binding to the rDNA promoter and rRNA synthesis. Overexpression of TIF-IA wild type (WT) but not the T340N mutant reduced the inhibitory effects of MPA on rRNA synthesis, cell proliferation, apoptosis, and p53 and p-H2AX expression, while increasing PCNA protein expression (Figure 3E and supplemental Figure 3E), further indicating the importance of GTP binding for TIF-IA function.
GTP binding is required for TIF-IA regulation of rRNA synthesis. (A) GTP binding motif in TIF-IA at amino acids 333 to 340 (top) and GTP binding by endogenous TIF-IA in primary T cells (bottom). T cells were isolated from PBMCs or were cultured in medium with anti-CD3/CD28. Lysates were incubated with GTP-agarose beads and the blots were probed with anti–TIF-IA antibody. (B) Effect of excess GTP on TIF-IA binding to GTP-agarose beads. Lysates from Jurkat or primary T cells were incubated with GTP-agarose beads in the presence or absence of GTP (10 mM). Blots were probed with anti–TIF-IA antibody. (C) Effect of a T340N mutation of TIF-IA on GTP binding. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA, and cultured for 24 hours. The lysates were incubated with GTP-agarose beads and blots were probed with anti-Myc antibody. (D) Effect of the T340N TIF-IA mutation on rRNA synthesis and Pol I binding to rDNA. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA. Lysate was incubated with anti-Myc antibody and a western blot of the precipitate probed with anti-Pol I antibody (left), densitometry measurements of Pol I binding are shown in supplemental Figure 3D, ChIP assay with Pol I antibody and western blot (middle), and 5′ETS pre-rRNA levels and RNA labeling (right). (E) Effect of TIF-IA or T340N TIF-IA overexpression on MPA-induced inhibition of rRNA synthesis and cell proliferation. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA and treated with MPA for 3 hours (left) or 24 hours (right). 5′ETS pre-rRNA and ChIP assay with Pol I antibody (left) and MTS assay, Annexin V staining, and western blot (right).
GTP binding is required for TIF-IA regulation of rRNA synthesis. (A) GTP binding motif in TIF-IA at amino acids 333 to 340 (top) and GTP binding by endogenous TIF-IA in primary T cells (bottom). T cells were isolated from PBMCs or were cultured in medium with anti-CD3/CD28. Lysates were incubated with GTP-agarose beads and the blots were probed with anti–TIF-IA antibody. (B) Effect of excess GTP on TIF-IA binding to GTP-agarose beads. Lysates from Jurkat or primary T cells were incubated with GTP-agarose beads in the presence or absence of GTP (10 mM). Blots were probed with anti–TIF-IA antibody. (C) Effect of a T340N mutation of TIF-IA on GTP binding. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA, and cultured for 24 hours. The lysates were incubated with GTP-agarose beads and blots were probed with anti-Myc antibody. (D) Effect of the T340N TIF-IA mutation on rRNA synthesis and Pol I binding to rDNA. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA. Lysate was incubated with anti-Myc antibody and a western blot of the precipitate probed with anti-Pol I antibody (left), densitometry measurements of Pol I binding are shown in supplemental Figure 3D, ChIP assay with Pol I antibody and western blot (middle), and 5′ETS pre-rRNA levels and RNA labeling (right). (E) Effect of TIF-IA or T340N TIF-IA overexpression on MPA-induced inhibition of rRNA synthesis and cell proliferation. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA and treated with MPA for 3 hours (left) or 24 hours (right). 5′ETS pre-rRNA and ChIP assay with Pol I antibody (left) and MTS assay, Annexin V staining, and western blot (right).
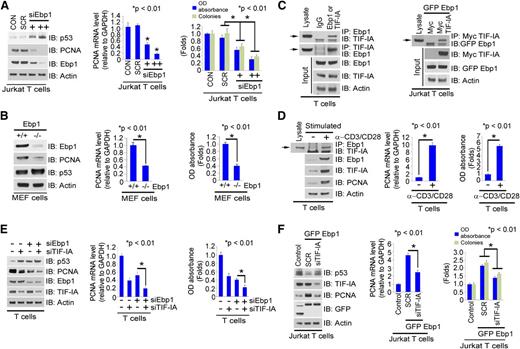
TIF-IA is a cofactor for Ebp1-regulated PCNA transcription
The above results suggested that MPA suppresses T-cell proliferation by decreasing the binding of GTP to TIF-IA, ultimately resulting in an increase in p53 and a decrease in PCNA expression. In looking for potential intermediates in this pathway, we examined the role of Ebp1, a negative regulator of p5323 that is increased in expression in proliferating T cells (Figure 2A). Overexpression of Ebp1 in Jurkat T cells decreased p53 expression and increased PCNA protein levels (supplemental Figure 4A, left), as has been noted previously.23 Ebp1 overexpression also enhanced cell proliferation and colony formation (supplemental Figure 4A, right), as well as PCNA messenger RNA (mRNA) synthesis (supplemental Figure 4A, middle). In contrast, reducing Ebp1 expression increased p53 expression while concomitantly decreasing PCNA mRNA and protein levels, and cell proliferation (Figure 4A). Comparing mouse embryonic fibroblasts (MEF) Ebp1−/− to Ebp1 WT cells,35 it is apparent that the absence of Ebp1 expression is associated with elevated p53 protein levels and decreased expression of PCNA at both the mRNA and protein levels (Figure 4B, left and middle). Overexpression of Ebp1 in MEF Ebp1−/− cells, which grow more poorly than their WT counterparts (Figure 4B, right), enhances both PCNA expression and cell proliferation (supplemental Figure 4B).
TIF-IA as a cofactor for Ebp1-regulated PCNA transcription. (A) Effects of Ebp1 depletion on PCNA expression and proliferation in Jurkat T cells. Cells were transfected with SCR or two concentrations of siEbp1 for 36 hours. Western blot with the indicated antibodies (left), PCNA mRNA level (middle), and MTS and colony forming assays (right). (B) Expression of Ebp1 in Ebp1+/+ and Ebp1−/− MEF cells. Western blot was probed with the indicated antibodies (left); PCNA mRNA level (middle) and MTS assay (right). (C) Interaction of Ebp1 and TIF-IA. T-cell lysate was incubated with anti-Ebp1 or TIF-IA antibody and western blots were probed with the antibodies shown (left) and Jurkat T cells were co-transfected with Myc vector alone or Myc–TIF-IA and GFP–Ebp1 (right). Cell lysate was incubated with anti-Myc antibody and the western blot probed with anti-GFP antibody. (D) Effect of T-cell activation with anti-CD3/CD28 on the interaction of Ebp1 and TIF-IA, PCNA expression, and proliferation. Lysate from resting or activated T cells was incubated with anti-Ebp1 antibody and the western blots were probed with the indicated antibodies (left), PCNA mRNA expression (middle), and MTS assay (right). (E) Effect of depletion of Ebp1, TIF-IA, or both in primary activated T cells on PCNA expression (left and middle) and cell proliferation (right). (F) Effect of Ebp1 overexpression in Jurkat T cells in the absence or presence of TIF-IA on PCNA expression (left and middle) and proliferation (right). The cells were pretreated with SCR or siTIF-IA for 24 hours and continuously transfected with GFP Ebp1 for an additional 24 hours.
TIF-IA as a cofactor for Ebp1-regulated PCNA transcription. (A) Effects of Ebp1 depletion on PCNA expression and proliferation in Jurkat T cells. Cells were transfected with SCR or two concentrations of siEbp1 for 36 hours. Western blot with the indicated antibodies (left), PCNA mRNA level (middle), and MTS and colony forming assays (right). (B) Expression of Ebp1 in Ebp1+/+ and Ebp1−/− MEF cells. Western blot was probed with the indicated antibodies (left); PCNA mRNA level (middle) and MTS assay (right). (C) Interaction of Ebp1 and TIF-IA. T-cell lysate was incubated with anti-Ebp1 or TIF-IA antibody and western blots were probed with the antibodies shown (left) and Jurkat T cells were co-transfected with Myc vector alone or Myc–TIF-IA and GFP–Ebp1 (right). Cell lysate was incubated with anti-Myc antibody and the western blot probed with anti-GFP antibody. (D) Effect of T-cell activation with anti-CD3/CD28 on the interaction of Ebp1 and TIF-IA, PCNA expression, and proliferation. Lysate from resting or activated T cells was incubated with anti-Ebp1 antibody and the western blots were probed with the indicated antibodies (left), PCNA mRNA expression (middle), and MTS assay (right). (E) Effect of depletion of Ebp1, TIF-IA, or both in primary activated T cells on PCNA expression (left and middle) and cell proliferation (right). (F) Effect of Ebp1 overexpression in Jurkat T cells in the absence or presence of TIF-IA on PCNA expression (left and middle) and proliferation (right). The cells were pretreated with SCR or siTIF-IA for 24 hours and continuously transfected with GFP Ebp1 for an additional 24 hours.
Ebp1 binds to TIF-IA in both primary and Jurkat T cells (Figure 4C). This interaction was confirmed by immunoprecipitation using recombinant GST–TIF-IA and endogenous Ebp1 or exogenous green fluorescent protein (GFP)-Ebp1 expressed in 293T cells (supplemental Figure 4C). The Ebp1–TIF-IA interaction is markedly enhanced in T cells stimulated with anti-CD3/CD28 antibodies (Figure 4D, left and right) and is associated with increases in PCNA mRNA and protein levels (Figure 4D, left and middle), suggesting that the interaction of TIF-IA with Ebp1 is important in regulating PCNA transcription through p53. Indeed, a reduction of either TIF-IA or the Ebp1 protein effectively increased p53 protein expression and inhibited PCNA transcription and cell proliferation (Figure 4E), whereas knocking down both Ebp1 and TIF-IA significantly enhanced the effects of siEbp1 alone (Figure 4E). In contrast, co-overexpression of Ebp1 and TIF-IA markedly decreased p53, and enhanced PCNA and cell proliferation (supplemental Figure 4D). Moreover, reducing the expression of TIF-IA diminished the effects of Ebp1 on both p53 expression, and PCNA transcription and cell proliferation (Figure 4F). These data demonstrate the relevance of the TIF-IA–Ebp1 interaction for the regulation of PCNA expression and cell proliferation, and also suggest a role for p53 as an intermediary in these effects.
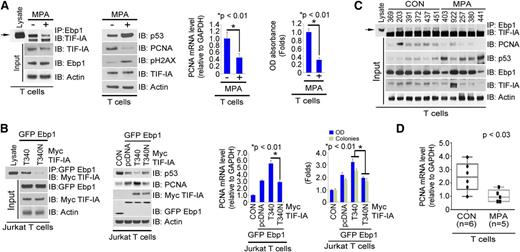
MPA treatment inhibits PCNA transcription by interfering with the TIF-IA–Ebp1 interaction
Treatment of primary T cells with MPA did not affect either TIF-IA or Ebp1 protein levels but decreased PCNA mRNA and protein expression (supplemental Figure 5A). Since TIF-IA binds to Ebp1 and is a cofactor with Ebp1 in the regulation of PCNA transcription (Figure 4), we hypothesized that MPA might interfere with the interaction of TIF-IA with Ebp1. Indeed, MPA treatment of both primary and Jurkat T cells diminished the TIF-IA–Ebp1 interaction, while increasing p53 and decreasing PCNA mRNA and protein expression and cell proliferation (Figure 5A and supplemental Figure 5B). These results suggested that the functional interaction of TIF-IA with Ebp1 might require TIF-IA to bind to GTP. Figure 5B (left) shows that the T340N TIF-IA mutant failed to bind Ebp1, whereas overexpression of T340N TIF-IA with Ebp1 failed to increase PCNA expression or cell proliferation (Figure 5B). The T340N mutant was also less effective than TIF-WT in enhancing the effects of Ebp1 on PCNA transcription (Figure 5B, near right). The interaction of TIF-IA with Ebp1, as well as PCNA protein and mRNA expression, were markedly reduced in cells from individuals treated with MPA (Figure 5C, first lane; Figure 5D; supplemental Figure 5C, left; and supplemental Figure 5D), whereas p53 expression was increased (Figure 5C, second and third lanes and supplemental Figure 5C, right).
Effects of MPA treatment on the interaction of TIF-IA and Ebp1 and the regulation of PCNA expression in T cells. (A-B) Effects of MPA on the interaction of TIF-IA and Ebp1 and PCNA levels in cultured primary T cells. Cells were treated with MPA for 24 hours. (A) Immunoprecipitation with anti-Ebp1 antibody (far left), immunoblotting with the antibodies indicated (near left), and PCNA expression and MTS assay (near right and far right). (B) Effects of the T340N mutation on the interaction of TIF-IA and Ebp1 and PCNA expression. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA and GFP Ebp1. Immunoprecipitation with anti-GFP antibody (far left), expression of p53 and PCNA (near left), PCNA mRNA expression (near right), and MTS and colony forming assay (far right). (C) Interaction of TIF-IA and Ebp1 and expression of PCNA in primary T cells from controls or MPA-treated individuals. Primary T cells were isolated from PBMCs of healthy donors (n = 6) or MPA-treated patients (n = 5). Lysate was incubated with anti-Ebp1 antibody and the precipitate blotted for TIF-IA. (D) PCNA mRNA expression.
Effects of MPA treatment on the interaction of TIF-IA and Ebp1 and the regulation of PCNA expression in T cells. (A-B) Effects of MPA on the interaction of TIF-IA and Ebp1 and PCNA levels in cultured primary T cells. Cells were treated with MPA for 24 hours. (A) Immunoprecipitation with anti-Ebp1 antibody (far left), immunoblotting with the antibodies indicated (near left), and PCNA expression and MTS assay (near right and far right). (B) Effects of the T340N mutation on the interaction of TIF-IA and Ebp1 and PCNA expression. Jurkat T cells were transfected with Myc–TIF-IA or Myc–T340N–TIF-IA and GFP Ebp1. Immunoprecipitation with anti-GFP antibody (far left), expression of p53 and PCNA (near left), PCNA mRNA expression (near right), and MTS and colony forming assay (far right). (C) Interaction of TIF-IA and Ebp1 and expression of PCNA in primary T cells from controls or MPA-treated individuals. Primary T cells were isolated from PBMCs of healthy donors (n = 6) or MPA-treated patients (n = 5). Lysate was incubated with anti-Ebp1 antibody and the precipitate blotted for TIF-IA. (D) PCNA mRNA expression.
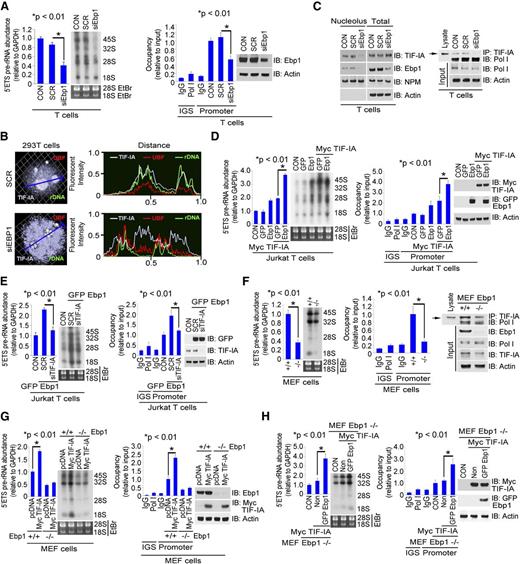
Ebp1 enhances rRNA synthesis through TIF-IA
We next asked whether the TIF-IA–Ebp1 interaction was important in the regulation of rRNA synthesis. Depletion of Ebp1 in primary T cells reduced rRNA synthesis and Pol I promoter occupancy (Figure 6A and supplemental Figure 6A) while increasing the translocation of TIF-IA from the nucleolus to the nucleus (Figure 6B and supplemental Figure 6B), suggesting that Ebp1 might be important for the retention of TIF-IA at the site of rRNA synthesis in the nucleolus. In cell fractionation experiments on primary T cells, the depletion of Ebp1 decreased the amount of TIF-IA in the nucleolus but did not noticeably affect the amount in total cell lysate (Figure 6C, left). Depletion of Ebp1 also decreased the interaction between TIF-IA and Pol I (Figure 6C, right). Co-overexpression of both TIF-IA and Ebp1 strongly enhanced both rRNA synthesis and Pol I recruitment to rDNA (Figure 6D), suggesting that both proteins participate in the regulation of rRNA synthesis, whereas the depletion of TIF-IA disrupted the enhancement of rRNA synthesis that occurred with Ebp1 expression (Figure 6E). As further confirmation of the requirement for both proteins, MEF Ebp1−/− cells demonstrated a low degree of interaction between TIF-IA and Pol I, and low levels of rRNA synthesis (Figure 6F). Overexpression of TIF-IA increased rRNA synthesis in MEF WT cells but not in MEF Ebp1−/− cells (Figure 6G), whereas co-expression of both TIF-IA and Ebp1 in MEF−/− cells strongly enhanced both TIF-IA binding to Pol I and rRNA synthesis (Figure 6H and supplemental Figure 6C). Thus, both proteins are directly involved in regulating rRNA levels.
Enhancement of rRNA synthesis by co-expression of TIF-IA with Ebp1. (A) Effects of Ebp1 depletion on rRNA synthesis in T cells. Cultured T cells were transfected with SCR or siEbp1 (20 nM) for 36 hours. 5′ETS pre-rRNA levels and RNA labeling (left) and ChIP assay with Pol I antibody and western blot (right). (B) Effects of Ebp1 depletion on TIF-IA localization; 293T cells were transfected with SCR or siEbp1 (20 nM) for 36 hours. Cells were co-stained with anti–TIF-IA and anti-UBF antibodies (left). rDNA was labeled with a rDNA probe as described in “Methods.” Fluorescence intensity was measured along the line through 3D pictures on the left (right). (C) Effects of Ebp1 depletion on TIF-IA binding with Pol I in primary T cells. Cells were transfected with SCR or siEbp1 (20 nM) for 36 hours. Nucleoli were isolated (see “Methods”) and nucleolar or whole cell lysates were immunoblotted for TIF-IA, Ebp1, and NPM1 (left), whereas cell lysate was incubated with anti–TIF-IA and the precipitate immunoblotted with anti-Pol I antibody (right). (D) Effects of co-overexpression of TIF-IA and Ebp1 on rRNA synthesis. Jurkat T cells were co-transfected with GFP-Ebp1 and vector control or Myc–TIF-IA for 24 hours. 5′ETS pre-rRNA and RNA labeling (left) and Pol I binding by ChIP assay and western blot (right). (E) Effects of TIF-IA depletion on Ebp1-enhanced rRNA synthesis. Jurkat T cells were co-transfected with GFP-Ebp1 and SCR or siTIF-IA (20 nM) for 36 hours. 5′ETS pre-rRNA and RNA labeling (left) and Pol I binding and western blot (right). (F) Effects of Ebp1 depletion on rRNA synthesis and Pol I binding to rDNA in MEF cells. RNA and protein were extracted from MEF–Ebp1+/+ or MEF–Ebp1−/− cells. 5′ETS pre-rRNA levels and RNA labeling (left), ChIP assay with Pol I antibody (middle), and TIF-IA was immunoprecipitated and western blot probed with anti-Pol I antibody (right). (G) Comparison of effects of TIF-IA overexpression in MEF–Ebp1+/+ and MEF–Ebp1−/− cells. Cells were transfected with vector control or TIF-IA for 24 hours. 5′ETS pre-rRNA levels and RNA labeling (left) and ChIP assay and western blot (right). (H) Effects of co-overexpression of Ebp1 and TIF-IA in MEF–Ebp1−/− cells. Cells were transfected with TIF-IA alone or co-transfected with TIF-IA and Ebp1 for 24 hours. 5′ETS pre-rRNA levels and RNA labeling (left) and ChIP assay and western blot (right).
Enhancement of rRNA synthesis by co-expression of TIF-IA with Ebp1. (A) Effects of Ebp1 depletion on rRNA synthesis in T cells. Cultured T cells were transfected with SCR or siEbp1 (20 nM) for 36 hours. 5′ETS pre-rRNA levels and RNA labeling (left) and ChIP assay with Pol I antibody and western blot (right). (B) Effects of Ebp1 depletion on TIF-IA localization; 293T cells were transfected with SCR or siEbp1 (20 nM) for 36 hours. Cells were co-stained with anti–TIF-IA and anti-UBF antibodies (left). rDNA was labeled with a rDNA probe as described in “Methods.” Fluorescence intensity was measured along the line through 3D pictures on the left (right). (C) Effects of Ebp1 depletion on TIF-IA binding with Pol I in primary T cells. Cells were transfected with SCR or siEbp1 (20 nM) for 36 hours. Nucleoli were isolated (see “Methods”) and nucleolar or whole cell lysates were immunoblotted for TIF-IA, Ebp1, and NPM1 (left), whereas cell lysate was incubated with anti–TIF-IA and the precipitate immunoblotted with anti-Pol I antibody (right). (D) Effects of co-overexpression of TIF-IA and Ebp1 on rRNA synthesis. Jurkat T cells were co-transfected with GFP-Ebp1 and vector control or Myc–TIF-IA for 24 hours. 5′ETS pre-rRNA and RNA labeling (left) and Pol I binding by ChIP assay and western blot (right). (E) Effects of TIF-IA depletion on Ebp1-enhanced rRNA synthesis. Jurkat T cells were co-transfected with GFP-Ebp1 and SCR or siTIF-IA (20 nM) for 36 hours. 5′ETS pre-rRNA and RNA labeling (left) and Pol I binding and western blot (right). (F) Effects of Ebp1 depletion on rRNA synthesis and Pol I binding to rDNA in MEF cells. RNA and protein were extracted from MEF–Ebp1+/+ or MEF–Ebp1−/− cells. 5′ETS pre-rRNA levels and RNA labeling (left), ChIP assay with Pol I antibody (middle), and TIF-IA was immunoprecipitated and western blot probed with anti-Pol I antibody (right). (G) Comparison of effects of TIF-IA overexpression in MEF–Ebp1+/+ and MEF–Ebp1−/− cells. Cells were transfected with vector control or TIF-IA for 24 hours. 5′ETS pre-rRNA levels and RNA labeling (left) and ChIP assay and western blot (right). (H) Effects of co-overexpression of Ebp1 and TIF-IA in MEF–Ebp1−/− cells. Cells were transfected with TIF-IA alone or co-transfected with TIF-IA and Ebp1 for 24 hours. 5′ETS pre-rRNA levels and RNA labeling (left) and ChIP assay and western blot (right).
Inhibition of T-cell activation and proliferation by combined treatment with MPA and sotrastaurin
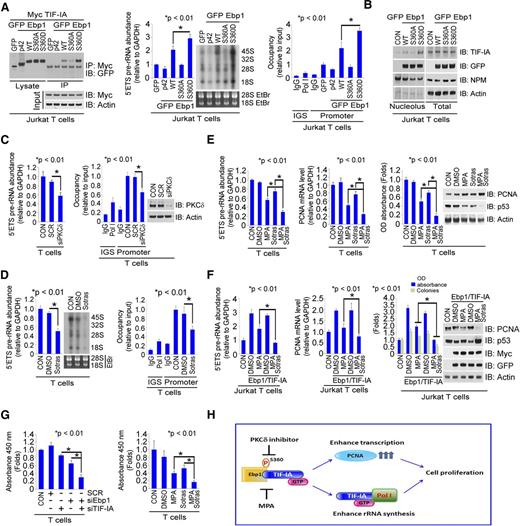
The phosphorylation of Ebp1 at serine 360 (S360) by protein kinase C δ (PKCδ) has been shown to increase cell proliferation in neuronal cells.27 We postulated that this specific posttranslational modification might also affect its interaction with TIF-IA. The transfection of a construct that substituted aspartic acid for serine at this position (S360D Ebp1) into Jurkat T cells demonstrated a stronger interaction of TIF-IA with the S360D mutant with Ebp1 WT, whereas a S360A Ebp1 mutant that cannot be phosphorylated at this site bound less tightly (Figure 7A, left). Overexpression of S360D but not S360A Ebp1 markedly enhanced rRNA synthesis (Figure 7A, middle and right) and retained more TIF-IA in the nucleolus (Figure 7B). These data suggest that the phosphorylation of Ebp1 by PKCδ at S360 is important for the regulation of rRNA synthesis by TIF-IA. In support of this concept, reducing PKCδ expression reduced the rRNA synthesis level in primary T cells (Figure 7C), as well as decreased the enhancement of rRNA synthesis induced by Ebp1 and TIF-IA overexpression in Jurkat T cells (supplemental Figure 7A).
Additive effects of MPA and sotrastaurin in inhibiting T-cell activation. (A) Effects of Ebp1 mutated to alanine at the S360 phosphorylation site on the regulation of rRNA synthesis. Interaction of TIF-IA with Ebp1–WT, Ebp1–S360A, and Ebp1–S360D. Jurkat T cells were co-transfected with the indicated constructs of GFP–Ebp1 and Myc–TIF-IA. Lysates were incubated with anti-Myc antibody and the precipitate immunoblotted with anti-GFP antibody (left). (Middle and right) Jurkat T cells were transfected with indicated constructs of Ebp1. 5′ETS pre-rRNA and RNA labeling (middle) and Pol I binding (right). (B) Effect of S360A–Ebp1 and S360D–Ebp1 expression on TIF-IA nucleolar localization. Jurkat T cells were transfected with WT–Ebp1, or S360A- and S360D-mutated Ebp1 constructs for 24 hours. Nucleoli were isolated and western blots were performed on nucleolar or whole cell lysate with the antibodies indicated. (C) Effects of PKCδ depletion on rRNA synthesis in T cells. Cells were transfected with SCR or siPKCδ (50 nM) for 36 hours. 5′ETS pre-rRNA levels (left), Pol I binding (middle), and PKCδ expression by western blot (right). (D) Effects of sotrastaurin on rRNA levels in T cells. The cells were treated with DMSO or sotrastaurin (100 nM) for 3 hours. 5′ETS pre-rRNA and RNA labeling (left) and Pol I binding assay (right). (E) Effects of combined treatment with MPA and sotrastaurin on rRNA synthesis, PCNA mRNA levels, and proliferation in T cells. Cultured T cells were treated with DMSO, MPA (100 nM), sotrastaurin (100 nM), or both for 24 hours. 5′ETS pre-rRNA levels (far left), PCNA mRNA level (near left), MTS assay (near right), and western blot (far right). (F) Effects of combined treatment with MPA and sotrastaurin on rRNA synthesis and proliferation with overexpression of TIF-IA and Ebp1. Jurkat T cells were co-transfected with Ebp1 and TIF-IA for 24 hours and then treated as shown. 5′ETS pre-rRNA levels (far left), PCNA mRNA level (near left), MTS and colony forming assay (near right), and western blot (far right). (G) Inhibition of IL-2 secretion by T cells with depletion of Ebp1 and TIF-IA (left) or with treatment with MPA and sotrastaurin (right). Cells were co-transfected with Ebp1 or TIF-IA siRNA (left) or treated with indicated drugs for 24 hours (right). IL-2 levels were measured by enzyme-linked immunosorbent assay. The samples were run in triplicate. (H) Schematic model of the effects of TIF-IA and Ebp1 and of MPA and sotrastaurin on the regulation of rRNA synthesis during T-cell activation.
Additive effects of MPA and sotrastaurin in inhibiting T-cell activation. (A) Effects of Ebp1 mutated to alanine at the S360 phosphorylation site on the regulation of rRNA synthesis. Interaction of TIF-IA with Ebp1–WT, Ebp1–S360A, and Ebp1–S360D. Jurkat T cells were co-transfected with the indicated constructs of GFP–Ebp1 and Myc–TIF-IA. Lysates were incubated with anti-Myc antibody and the precipitate immunoblotted with anti-GFP antibody (left). (Middle and right) Jurkat T cells were transfected with indicated constructs of Ebp1. 5′ETS pre-rRNA and RNA labeling (middle) and Pol I binding (right). (B) Effect of S360A–Ebp1 and S360D–Ebp1 expression on TIF-IA nucleolar localization. Jurkat T cells were transfected with WT–Ebp1, or S360A- and S360D-mutated Ebp1 constructs for 24 hours. Nucleoli were isolated and western blots were performed on nucleolar or whole cell lysate with the antibodies indicated. (C) Effects of PKCδ depletion on rRNA synthesis in T cells. Cells were transfected with SCR or siPKCδ (50 nM) for 36 hours. 5′ETS pre-rRNA levels (left), Pol I binding (middle), and PKCδ expression by western blot (right). (D) Effects of sotrastaurin on rRNA levels in T cells. The cells were treated with DMSO or sotrastaurin (100 nM) for 3 hours. 5′ETS pre-rRNA and RNA labeling (left) and Pol I binding assay (right). (E) Effects of combined treatment with MPA and sotrastaurin on rRNA synthesis, PCNA mRNA levels, and proliferation in T cells. Cultured T cells were treated with DMSO, MPA (100 nM), sotrastaurin (100 nM), or both for 24 hours. 5′ETS pre-rRNA levels (far left), PCNA mRNA level (near left), MTS assay (near right), and western blot (far right). (F) Effects of combined treatment with MPA and sotrastaurin on rRNA synthesis and proliferation with overexpression of TIF-IA and Ebp1. Jurkat T cells were co-transfected with Ebp1 and TIF-IA for 24 hours and then treated as shown. 5′ETS pre-rRNA levels (far left), PCNA mRNA level (near left), MTS and colony forming assay (near right), and western blot (far right). (G) Inhibition of IL-2 secretion by T cells with depletion of Ebp1 and TIF-IA (left) or with treatment with MPA and sotrastaurin (right). Cells were co-transfected with Ebp1 or TIF-IA siRNA (left) or treated with indicated drugs for 24 hours (right). IL-2 levels were measured by enzyme-linked immunosorbent assay. The samples were run in triplicate. (H) Schematic model of the effects of TIF-IA and Ebp1 and of MPA and sotrastaurin on the regulation of rRNA synthesis during T-cell activation.
Sotrastaurin, a protein kinase inhibitor, is used to prevent transplant rejection and to treat certain inflammatory diseases.36 Sotrastaurin inhibits rRNA synthesis in primary T cells (Figure 7D) and in Jurkat T cells in which Ebp1 and TIF-IA are co-expressed (supplemental Figure 7B). To determine whether the effects of sotrastaurin on rRNA synthesis are mediated by the inhibition of PKCδ phosphorylation, we overexpressed Myc–TIF-IA in conjunction with either Ebp1 WT or Ebp1 S360D. Sotrastaurin inhibited both the binding of TIF-IA to Pol I and rRNA synthesis in the presence of WT Ebp1 but not of the S360D Ebp1 mutant (supplemental Figure 7C). The combination of sotrastaurin with MPA inhibited rRNA synthesis, PCNA mRNA expression, and cell proliferation to a greater extent than either agent alone in primary activated T cells (Figure 7E) and in Jurkat T cells overexpressing Ebp1 and TIF-IA (Figure 7F). Finally, reducing the expression of both Ebp1 and TIF-IA, compared with either alone or in combination with MPA and sotrastaurin as opposed to either drug alone, markedly reduced the production of IL-2 by primary T cells (Figure 7G). In summary, inhibiting Ebp1 phosphorylation at S360 with an inhibitor of PKCδ appears to be an effective mechanism for inhibiting T-cell activation and proliferation, and is complementary to the inhibitory effects of MPA on rRNA synthesis.
Discussion
The synthesis of rRNA, an essential component of ribosome biogenesis, is regulated in a growth-dependent manner that is evolutionarily conserved. TIF-IA is one of the critical components of the pre-initiation complex that is required for the transcription of rDNA into rRNA, and is thus a key target for growth-regulated signaling.37 Although it has been recognized that TIF-IA contains a Walker-type A- or P-loop motif,37,38 the significance of this sequence for TIF-IA function had not been explored. We have demonstrated that TIF-IA is a GTP binding protein and that depletion of GTP by MPA, an inhibitor of de novo guanine nucleotide synthesis, as well as mutation of the Walker-A sequence at T340 inhibit rRNA synthesis. These findings demonstrate that TIF-IA requires GTP to function in the transcription initiation complex required for rRNA synthesis. Treatment with MPA simultaneously induces the translocation of TIF-IA to the periphery of the nucleolus and blocks the recruitment of Pol I to rDNA in primary T cells, demonstrating its dependence on intracellular GTP pools. These findings provide a molecular basis for the early observations that intracellular pool sizes of ATP and GTP are critical for rRNA synthesis in mouse cells.39
The two isoforms of Ebp1, p48 and p42,22 have distinct subcellular localizations and functions. The p48 isoform contains an additional 54 amino acids at the amino terminus and promotes cell proliferation,23,40 whereas the shorter p42 form is frequently ubiquitinated and degraded and is rarely detected on immunoblots.23,41 In both primary T cells and Jurkat T cells, only the 48 kDa protein was detectable on western blots. We found that the p48 isoform interacts with TIF-IA to enhance both rRNA synthesis and proliferation in T cells (Figure 7A).
Ebp1 has been shown to enhance cell growth and proliferation in diverse cell types by interacting with many protein partners. As examples, the interaction of Ebp1 with protein kinase B/Akt prevented apoptotic cell death and enhanced survival in neuronal cells27 ; the association of Ebp1 with nucleophosmin was shown to be essential for cell proliferation and cell survival in rat PC12 cells28 ; the binding of Ebp1 to Hdm2 promoted the Hdm2-induced ubiquitination and subsequent degradation of p53, promoting tumorigenesis in human glioma cells23 ; and, most recently, binding of Ebp1 to cyclin-dependent kinase 2 resulted in Ebp1 phosphorylation and promotion of tumor formation.40 Thus, an abundance of data supports a role for p48 Ebp1 in tumorigenesis as a result of its interactions with multiple protein partners. Our results demonstrate a previously unreported mechanism by which Ebp1 promotes cell proliferation, specifically through increasing the transcription of rRNA and of PCNA through its interaction with TIF-IA.
Many elements control the transcription of the PCNA gene,42 which is both positively and negatively regulated as cells progress through the cell cycle. One such regulator is p53. In cells exposed to genotoxic stress, p53 expression correlates both directly43-46 and inversely47 with PCNA expression. Previous studies have demonstrated that high levels of p53 expression increase the binding of p53 to the PCNA promoter and decrease the extent of H4 acetylation, resulting in the inhibition of PCNA transcription. In contrast, a decrease in p53 expression enhances PCNA transcription.42,48-50 Our results suggest that TIF-IA may be a cofactor with Ebp1 in reducing p53 expression. Ebp1 has been shown to bind to the E3 ligase HDM2, enhancing the HDM2–p53 association and promoting p53 polyubiquitination and degradation.23,24 As shown in our study, the knockdown of TIF-IA or dissociation of the interaction of TIF-IA with Ebp1 increases p53 expression, while decreasing PCNA expression and cell proliferation. Taken together, our results suggest a model whereby the TIF-IA–Ebp1 interaction may regulate the proliferation of T cells by controlling PCNA expression, possibly through p53 (Figure 7H).
PKC has been shown to be important in the regulation of immune cell function,51,52 and represents a potentially important target for the treatment of allograft rejection and autoimmune diseases. Sotrastaurin is a low-molecular weight molecule that has been studied as a potential immunosuppressive agent that targets both classical (α, β) and novel (δ, ε, η, θ) PKC isoforms, and inhibits CD3/CD28 antibody- and alloantigen-induced T-cell proliferative responses in vitro.53 Phase 2 clinical trials have shown that sotrastaurin has efficacy in psoriasis and in renal transplantation.54,55 Based on our results, we postulate that the efficacy of sotrastaurin relates, at least in part, to the inhibition of rRNA synthesis in T cells by dissociating Ebp1 from TIF-IA. As a consequence of the phosphorylation of Ebp1 at S360 by PKCδ, Ebp1 interacts with Akt in neuronal cells27 and binds more strongly to nucleophosmin.28 Although sotrastaurin is a pan-PKC inhibitor and may have effects within the cell that extend beyond the Ebp1–TIF-IA interaction, the fact that the S360D mutant largely abrogates the ability of sotrastaurin to inhibit rRNA synthesis supports specificity for S360 phosphorylation as the primary mechanism by which the drug is having its effect. We conclude that the Ebp1–TIF-IA interaction is also dependent on S360 phosphorylation.
Over the past 2 decades, the success of various forms of transplantation has been tightly linked to the development of new approaches to immunosuppression.56,57 Recent advances frequently require the combination of several agents with complementary mechanisms of action. The finding that the combination of MPA and sotrastaurin has additive effects in inhibiting both rRNA synthesis and T-cell activation while simultaneously decreasing PCNA expression (Figure 7H) suggests that this combination might have enhanced clinical efficacy over MMP alone for autoimmune and other disorders that result from T lymphocyte activation.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a translational research grant and by a Specialized Center of Research award from the Leukemia and Lymphoma Society, and a grant from the National Institutes of Health National Cancer Institute (1RO1CA138583) (A.W.H.).
Authorship
Contribution: L.X.T.N. and B.S.M. designed research; L.X.T.N., Y.L., and L.U. performed research; L.X.T.N., J.B.S., and B.S.M. analyzed data; P.J.U. and A.W.H. supplied the agents; and L.X.T.N. and B.S.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no completing financial interests.
Correspondence: Beverly S. Mitchell, Lorry Lokey Building, 265 Campus Dr, Suite G2167, Stanford, CA 94305-5456; e-mail: bmitchell@stanford.edu.

![Figure 1. Inhibition of rRNA synthesis and cell proliferation in T cells by MPA. (A) Effects of MPA on rRNA synthesis and cell proliferation in cultured primary T cells. Primary T cells were isolated from PBMCs of healthy donor blood and cultured in stimulation medium (see “Methods”). (Left and middle) Cells were treated with DMSO or MPA (100 nM) for 3 hours. RNA was extracted for measurement of 5′ETS pre-rRNA and RNA was labeled with [32P] (left). ChIP assay was performed to measure the level of Pol I recruited to rDNA promoter (middle). GAPDH (left) or 10% input (middle) were used as internal controls and samples were run in triplicate. Cells were cultured in proliferation medium in the presence of DMSO or MPA (100 nM) for 24 hours. MTS assay and western blot were performed as shown (right). (B) Effects of MPA on rRNA synthesis and cell proliferation in primary T cells from patients treated with MPA. T cells were isolated from PBMCs of healthy donors (n = 6) or individuals treated with MPA (n = 5). RNA was extracted for the measurement of 5′ETS pre-rRNA (left), ChIP assay was performed to measure the level of Pol I recruited to rDNA promoter (middle), and MTS assay and western blot were performed as shown (right). Significance was determined using the Student t test. Individual values are shown in supplemental Figure 1B. CON, control; EtBr, ethidium bromide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IGS, intergenic spacer; OD, optical density.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/16/10.1182_blood-2014-12-616433/4/m_2519f1.jpeg?Expires=1765901171&Signature=lfnczQrt0fUmFJHb-KVABIaH-VYjwY6qA7Q3UKN1IzO3HiCVcBsRFUXvMKZIDnMucNlm--xM1Ev1sf3Eav4e78to00jZ3Ypq~i4dJM49Ev0BvaUvkyL~w585LLPV282Mzfmt0vhv8HLz2kfUJ8WSolfu8e~yT~pL7PyCBjO15CjqoDjiswq6yA7xV-Hb601viIUTS7SX4zGh5NBtZkSrhCI~dP469pMcAxoKd0JsbD4n4F~uIzueXCm-Pm8yL01IKev040rJNbh8oBWuFfrfSSMzkFQO4rmuQ2FIhOct4YHV-suf7G45ctCo7wLK3~~5oMPtMmJdw0BHrNtevvKPoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Effects of MPA treatment on TIF-IA–regulated rRNA synthesis in T cells. (A) Expression of TIF-IA, PCNA, and Ebp1 protein in T cells stimulated for 5 days with anti-CD3 and anti-CD28. Primary T cells were isolated from PBMCs and cultured in control medium or medium containing anti-CD3/CD28. (B) Effects of TIF-IA depletion on rRNA synthesis and proliferation in primary T cells. Cells were cultured in proliferation medium and then transfected with SCR or siTIF-IA (20 nM) for 36 hours. RNA was extracted for measurement of 5′ETS pre-rRNA (left), Pol I recruitment to the rDNA promoter (middle), and MTS assay and western blot were performed as shown (right). (C) Effects of MPA on TIF-IA regulation of rRNA synthesis. Primary T cells were cultured in proliferation medium and treated with MPA for the indicated times. TIF-IA was immunoprecipitated from cell lysate and blots were probed with anti-Pol I antibody (left), ChIP assay with Pol I antibody (middle), and 5′ETS pre-rRNA levels and RNA labeling with [32P] (right). (D) Effects of MPA on TIF-IA binding with Pol I in cells from individuals treated with MPA. TIF-IA was immunoprecipitated from cell lysate that was pooled from healthy donors (n = 6) or individuals treated with MPA (n = 5), and blots were probed with anti-Pol I antibody. (E) Effects of TIF-IA depletion on rRNA synthesis and cell proliferation in T cells treated with MPA. The cells were transfected with SCR or siTIF-IA (20 nM) for 24 hours, then treated with MPA (100 nM) for 3 hours (left and middle) or 24 hours (right). 5′ETS pre-rRNA (left), ChIP assay (middle), and MTS and western blot (right).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/16/10.1182_blood-2014-12-616433/4/m_2519f2.jpeg?Expires=1765901171&Signature=hYpg5htv5UNJ4SyxbR82degseEEGmim74mnSZ9xutJwpJ9plZUPqyMmTQGAVWoasUwvcbwQVGcH8iYha5dwwyIZ7rZIUHMdpryP9ZOmIEWV2cTygs94C7-PnT9t1Dz3GjaySZR~Mw3lqG8-EzkRtk~nyujE7YFCGsN9qeiiA5d4TUcXLQYsWbbsSRX-xyNu7dkDhT~CHz~wf3gYHnB93~TWu6NWBAorSsNvmemdUw4252u51YVjgUDWEZRfdcv0YWZ~BSS7tLvtTIliVZamnS5G0xmzlumm6epq2y2S2c~ZmIOn3Mnnjshlt4BHkfvGNXr1-1UYa5wUzhmKP6YSTog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)