Key Points
Highly elevated ferritin is not specific for hemophagocytic lymphohistiocytosis in adults.
Marked hyperferritinemia in adults most often occurs in the setting of renal failure, hepatocellular injury, infection, or malignancy.
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare syndrome of uncontrolled immune activation that has gained increasing attention during the last decade. The diagnosis of HLH is based on a constellation of clinical and laboratory abnormalities, including elevated serum ferritin levels. In the pediatric population, marked hyperferritinemia is specific for HLH. To determine what conditions are associated with profoundly elevated ferritin in the adult population, we performed a retrospective analysis in a large academic health care system. We identified 113 patients with serum ferritin levels higher than 50 000 µg/L. The most frequently observed conditions included renal failure, hepatocellular injury, infections, and hematologic malignancies. Our results suggest that marked hyperferritinemia can be seen in a variety of conditions and is not specific for HLH in adults.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare and often fatal syndrome of immune overactivation. The disorder is caused by genetic mutations affecting cytotoxic function (familial HLH) or secondary to infectious, rheumatologic, or malignant conditions (acquired HLH).1-7 Although hyperferritinemia can be seen in a variety of conditions, markedly elevated serum ferritin concentrations are often thought to be exclusive to rheumatologic and inflammatory disorders, including adult-onset Still disease (AOSD), HLH, and the related macrophage activation syndrome (MAS). In the pediatric population, extremely elevated ferritin is highly sensitive and specific for HLH. A retrospective analysis at Texas Children’s Hospital performed by Allen et al reported that a maximum ferritin level higher than 10 000 μg/L had a 90% sensitivity and 98% specificity for HLH.8 This is important because the clinical presentation of HLH is nonspecific, and treatment is often delayed because of uncertainty in diagnosis. The assay for ferritin is universally available, and results are returned relatively quickly, further increasing the clinical use of ferritin as a test for rapid diagnosis. In adults, a moderate degree of ferritin elevation can be seen in a variety of disorders, including HLH, AOSD, iron overload, liver failure, malignancy, infection, renal failure, antiphospholipid syndrome, and anemia of chronic disease.9-17 To determine what conditions are associated with profoundly elevated ferritin levels in the adult population, we performed a retrospective analysis of patients with marked hyperferritinemia in a large academic health care system.
Methods
Institutional review board approval was requested to search the data repository of the Partners Healthcare System for patients with elevated ferritin or hemophagocytic lymphohistiocytosis. Approval was granted through the Partners Healthcare institutional review board. Using the Research Patient Data Registry (RPDR), a centralized clinical data registry, we searched for all patients at Brigham and Women’s Hospital (BWH), Massachusetts General Hospital (MGH), and Faulkner Hospital (FH) who had serum ferritin levels above various thresholds between August 1, 1988, and January 29, 2014, for BWH and MGH, and August 9, 1995, and January 29, 2014, for FH (Table 1). Surprisingly, more than 800 individuals were identified using the previously described cutoff of more than 10 000 μg/L, suggesting this value would not be specific for HLH, which is a rare disorder, in this population. Using the higher ferritin value of more than 50 000 µg/L identified only 111 patients (±3 patients, the RPDR error margin on initial query). Allen et al found that both admission and maximum ferritin levels were highest in patients with HLH compared with all other diagnoses, suggesting a higher ferritin threshold might be more specific for HLH.8 Experience at our institution supports that HLH is enriched as the ferritin threshold increases, although the ferritin levels in documented HLH cases in our institutions ranged from less than 1000 to more than 50 000 (A.M.S., A.M., and N.B., unpublished data). In addition, based on the number of HLH cases at our institution and the number of patients identified with various ferritin thresholds, any value lower than 50 000 µg/L was unlikely to be specific for this diagnosis. Thus, we chose to more closely examine the charts of all patients with the higher ferritin threshold of more than 50 000 µg/L. For patients who had multiple ferritin levels higher than 50 000 µg/L, we used the highest value. Ferritin assays differed slightly depending on institution and date, but in all cases, our cutoff level of higher than 50 000 µg/L was more than 500 times the upper limit of normal. Patients younger than 18 years were excluded, and the medical charts of the remaining patients were manually reviewed.
Estimated number of patients with ferritin above a threshold through the RPDR system
| Ferritin (µg/L) . | N* . |
|---|---|
| >10 000 | 822 |
| >20 000 | 340 |
| >30 000 | 201 |
| >40 000 | 147 |
| >50 000 | 111 |
| >60 000 | 91 |
| >100 000 | 42 |
| >200 000 | 5 |
| Ferritin (µg/L) . | N* . |
|---|---|
| >10 000 | 822 |
| >20 000 | 340 |
| >30 000 | 201 |
| >40 000 | 147 |
| >50 000 | 111 |
| >60 000 | 91 |
| >100 000 | 42 |
| >200 000 | 5 |
RPDR returns patient number with an error margin of ±3 patients. When the records for patients with ferritin levels higher than 50 000 µg/L were requested for review, the system returned 113 records.
We collected demographic data including race, sex, and age at the time of elevated ferritin. Each patient was categorized as having HLH, MAS, rheumatologic/inflammatory disorder, hematologic malignancy, solid tumor, renal failure, infection, hepatocellular injury, iron overload, hemolytic anemia, or none of these conditions. Causes were not considered mutually exclusive. Patients were determined to have HLH on the basis of the HLH-2004 criteria, which require either a molecular diagnosis consistent with HLH or fulfillment of 5 of 8 criteria.4 These criteria include fever, splenomegaly, cytopenias affecting 2 or more lineages (hemoglobin <9 g/dL, platelets <100 × 109/L, neutrophils <1.0 × 109/L), hypertriglyceridemia and/or hypofibrinogenemia (≥265 mg/dL or ≤150 mg/dL, respectively), hemophagocytosis (in bone marrow, spleen, or lymph node), hyperferritinemia (ferritin ≥500 ng/mL), impaired natural killer cell function, and elevated CD25 (ie, soluble IL-2 receptor) of 2400 U/mL or higher. We evaluated all patients for the presence or absence of these factors. This included reviewing all notes, radiographic, and laboratory studies available on the electronic medical record. Patients were deemed not to have HLH if they had sufficient testing and did not meet criteria for HLH, or if there was no clinical suspicion for HLH and they therefore did not have adequate testing because of a lack of bone marrow biopsy, CD25 level, or natural killer cell function. Three patients were included who were clinically thought to have HLH and who met 4 of 8 criteria but did not have the remaining criteria checked. Patients were considered to have MAS if this constellation of signs and symptoms was present in the setting of a rheumatologic condition or a rheumatologist concluded the patient had MAS despite inadequate testing. One patient had both Epstein-Barr virus infection and AOSD and was recorded as having both HLH and MAS. Patients were categorized as having a malignancy if they had active cancer or had received cancer treatment in the previous 6 months. Malignancy was further subdivided into hematologic or solid tumor. Renal failure was defined as requiring renal replacement therapy or having a glomerular filtration rate lower than 20 mL/minute/1.73 m2. Patients were classified under infection if they had a severe infection requiring hospitalization or antimicrobials or a more chronic infection thought to be active and clinically relevant. This included bacterial, viral, and fungal sources of infection. Hepatocellular dysfunction was defined as having a primary acute liver process or severe liver damage thought to be significant, as documented by the providers, with alanine aminotransferase and aspartate aminotransferase typically higher than 500 U/L. Patients who were deemed to have iron overload had received monthly red blood cell transfusions for at least 6 months, required chelation therapy, or were determined by a hematologist to have iron overload.
Descriptive statistics were calculated using frequencies and percentages, along with their binomial exact 90% confidence intervals for categorical variables, and using range and median for continuous variables. Univariate and multivariable linear regressions were used to evaluate the association of those factors with ferritin levels modeled using the logarithmic transformation of the actual ferritin levels. The Wilcoxon rank sum test was also performed, along with the univariate analysis. Those covariates demonstrated to be statistically significant at an α level of 0.2 were included in the multivariable stepwise linear regression model. In the multivariable analysis, a P value <.05 was considered statistically significant.
Results
We identified 113 total patients with ferritin levels above 50 000 µg/L at BWH, MGH, and FH during the study period. The patients ranged from 20 to 88 years, with a median of 58 years (Table 2). The majority of patients were male (58%). The racial distribution included white (75%), black (16%), Hispanic (4%), Asian (2%), and unknown (3%).
Patient demographics
| Demographic . | Total . |
|---|---|
| Age, median [range] | 58 [20-88] |
| Sex, N (%) | |
| Male | 66 (58) |
| Female | 47 (42) |
| Race, N (%) | |
| White | 85 (75) |
| Black | 18 (16) |
| Hispanic | 5 (4) |
| Asian | 2 (2) |
| Unknown | 3 (3) |
| Demographic . | Total . |
|---|---|
| Age, median [range] | 58 [20-88] |
| Sex, N (%) | |
| Male | 66 (58) |
| Female | 47 (42) |
| Race, N (%) | |
| White | 85 (75) |
| Black | 18 (16) |
| Hispanic | 5 (4) |
| Asian | 2 (2) |
| Unknown | 3 (3) |
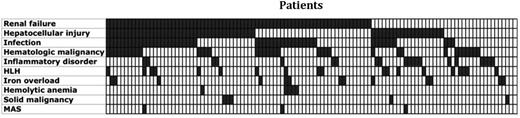
The diagnoses for the patients with ferritin levels higher than 50 000 µg/L included renal failure (65%), hepatocellular injury (54%), infection (46%), hematologic malignancy (32%), rheumatologic/inflammatory conditions (18%), HLH (17%), iron overload (12%), hemolytic anemia (4%), solid malignancy (4%), and MAS (3%) (Table 3). The majority of patients had more than one of the described conditions, with a median of 2 and range of 0 to 7 conditions. Two (2%) patients did not fit into any of these categories (Figure 1). Patients with renal insufficiency exhibited a range of acuity. Some had chronic kidney disease with no apparent acute insult, and others had acute or chronic kidney disease or acute kidney injury. Liver disease often occurred in the setting of malignancy, infection, or shock with multiorgan dysfunction syndrome; however, it was also seen as a primary disorder with fulminant liver failure secondary to Tylenol overdose or viral hepatitis. The spectrum of infections identified in this cohort was broad and included bacterial, viral, and fungal sources of infection. Hematologic malignancies were common, with the majority being of B-cell origin (16/36), followed by myeloid (13/36) and then T-cell origin (7/36). Five patients had a diagnosis of a solid tumor, including 1 with early-stage breast cancer, 1 with stage III breast and ovarian cancer, 2 with metastatic lung cancer, and 1 with stage IVa squamous cell carcinoma of the tongue. Inflammatory conditions included AOSD, systemic lupus erythematosus, catastrophic antiphospholipid antibody syndrome, psoriatic arthritis, and in multiple patients, undifferentiated rheumatic diseases and overlap syndromes.
Distribution of patients with elevated ferritin by disorder
| Disorder . | N (% [90% confidence interval]) . | Ferritin (×103 µg/L), median [range] . |
|---|---|---|
| Renal failure | 73 (65 [57-72]) | 84 [50-440] |
| Hepatocellular injury | 61 (54 [46-62]) | 83 [50-440] |
| Infection | 52 (46 [38-54]) | 87 [52-440] |
| Hematologic malignancy | 36 (32 [25-40]) | 98 [52-233] |
| Rheumatologic/inflammatory | 20 (18 [12-25]) | 68 [50-341] |
| HLH | 19 (17 [11-24]) | 99 [52-233] |
| Iron overload | 13 (12 [7-18]) | 101 [76-440] |
| Hemolytic anemia | 5 (4 [2-9]) | 149 [80-203] |
| Solid tumor | 5 (4 [2-9]) | 72 [52-95] |
| MAS | 3 (3 [1-7]) | 61 [56-341] |
| None | 2 (2 [0-4]) | 123 [118-127] |
| Disorder . | N (% [90% confidence interval]) . | Ferritin (×103 µg/L), median [range] . |
|---|---|---|
| Renal failure | 73 (65 [57-72]) | 84 [50-440] |
| Hepatocellular injury | 61 (54 [46-62]) | 83 [50-440] |
| Infection | 52 (46 [38-54]) | 87 [52-440] |
| Hematologic malignancy | 36 (32 [25-40]) | 98 [52-233] |
| Rheumatologic/inflammatory | 20 (18 [12-25]) | 68 [50-341] |
| HLH | 19 (17 [11-24]) | 99 [52-233] |
| Iron overload | 13 (12 [7-18]) | 101 [76-440] |
| Hemolytic anemia | 5 (4 [2-9]) | 149 [80-203] |
| Solid tumor | 5 (4 [2-9]) | 72 [52-95] |
| MAS | 3 (3 [1-7]) | 61 [56-341] |
| None | 2 (2 [0-4]) | 123 [118-127] |
Nineteen patients were found to have HLH. All were thought to have secondary HLH triggered by a malignancy (9/19) or infection (6/19), with the minority having no clear precipitating cause (4/19). All 9 patients with malignancy-associated HLH had a hematologic malignancy (4/9 had T-cell lymphoma, 3/9 had diffuse large B-cell lymphoma, 1/9 had transformed acute myeloid leukemia, and 1/9 had erythroleukemia). Ten patients with HLH also developed an associated acute kidney injury, and 10 patients developed acute hepatocellular injury. Of the 10 who developed acute hepatocellular injury, 2 had underlying mild hepatocellular dysfunction that acutely and significantly worsened during the hospitalization for HLH. Nearly all of the patients with HLH had fever (18/19), splenomegaly (16/18), and cytopenia in 2 or more cell lines (19/19). The assay for natural killer cell function was only sent in 1 of the patients (Table 4).
Distribution of clinical and laboratory abnormalities in patients with HLH
| Abnormalities . | N . | % [90% confidence interval] . |
|---|---|---|
| Fever | 18/19 | 95 [77-100] |
| Splenomegaly | 16/18 | 89 [69-98] |
| Cytopenia more than 1 cell line | 19/19 | 100 [85-100] |
| Anemia | 14/19 | 74 [52-89] |
| Thrombocytopenia | 19/19 | 100 [85-100] |
| Neutropenia | 19/19 | 100 [85-100] |
| Hypertriglyceridemia | 14/18 | 78 [56-92] |
| Hypofibrinogenemia | 10/19 | 53 [32-73] |
| sIL2-R elevation | 10/10 | 100 [74-100] |
| Low natural killer cell function | 0/1 | 0 [0-95] |
| Hemophagocytosis | 12/16 | 75 [52-91] |
| Abnormalities . | N . | % [90% confidence interval] . |
|---|---|---|
| Fever | 18/19 | 95 [77-100] |
| Splenomegaly | 16/18 | 89 [69-98] |
| Cytopenia more than 1 cell line | 19/19 | 100 [85-100] |
| Anemia | 14/19 | 74 [52-89] |
| Thrombocytopenia | 19/19 | 100 [85-100] |
| Neutropenia | 19/19 | 100 [85-100] |
| Hypertriglyceridemia | 14/18 | 78 [56-92] |
| Hypofibrinogenemia | 10/19 | 53 [32-73] |
| sIL2-R elevation | 10/10 | 100 [74-100] |
| Low natural killer cell function | 0/1 | 0 [0-95] |
| Hemophagocytosis | 12/16 | 75 [52-91] |
Of the 2 patients with ferritin elevation and none of the described conditions, 1 had cholecystitis in addition to possible sinusitis. The other was admitted for a non-ST-elevation myocardial infarction and was taken to 3-vessel coronary artery bypass grafting with a course complicated by heart failure. More than half of the patients received documented transfusions (58%), and many patients were thought to possibly receive transfusions at an outside institution before transfer or without electronic documentation (15%).
Ferritin levels ranged from 50 129 to 439 500 µg/L, with a median of 83 272 µg/L. One of the ferritin assays used during the study period did not quantify values higher than 100 000 µg/L, and 2 patients were documented as having a ferritin level higher than 100 000 µg/L. These patients were excluded from the disease-specific analyses, including the univariate and multivariable analyses. The highest median ferritin level was seen in patients with hemolytic anemia (Table 3). The patient with the highest ferritin level of 439 500 µg/L had end-stage renal disease on hemodialysis and presented with hypotension requiring vasopressors. The patient developed shock liver and ischemic colitis causing a gastrointestinal bleed that required transfusion, and had a skin lesion confirmed as mucormycosis.
The univariate analysis revealed that renal failure, infection, hematologic malignancy, rheumatologic/inflammatory disease, iron overload, hemolytic anemia, and solid malignancy were significant at an α level of 0.2. These characteristics were included in the multivariable analysis. The stepwise linear regression showed that presence of hemolytic anemia was the only variable associated with a differential ferritin level (β = 0.41; P = .03).
Discussion
Ferritin is a ubiquitous, 450-kDa, 24-subunit protein that is best known for its role in iron storage.18,19 The majority of ferritin is intracellular, where it functions to bind iron in a nontoxic form and release it in a controlled fashion. In addition to the well-studied cytosolic ferritin, this protein can be identified in the circulation.20 The expression and secretion of ferritin is tightly regulated at the transcriptional and posttranscriptional level by a variety of factors including iron, cytokines, hormones, and oxidative stress.17,21-23 In vivo, ferritin seems to be primarily released from macrophages.24,25 Serum ferritin levels typically reflect total body iron stores.26-28 Clinically, it is often measured to identify patients with iron deficiency or iron overload syndromes. A serum ferritin less than 10 to 15 µg/L is 99% specific for making a diagnosis of iron deficiency.29 In contrast, an elevated ferritin concentration can be seen in a variety of conditions including iron overload syndromes, infection, inflammatory disorders, malignancy, liver failure, and renal failure.9-17
In a large retrospective analysis at an academic medical center, Moore et al reviewed the charts of 627 adults with serum ferritin levels higher than 1000 µg/L between 2008 and 2010.9 Patients were categorized according to what was assumed to be the predominant cause of hyperferritinemia, using a predetermined algorithm. The average serum ferritin level was 2647 µg/L, with the most common conditions being malignancy (24%) and iron overload (22%). Few patients had HLH, MAS, or adult-onset Still disease (1%); however, this paper used the relatively low ferritin threshold of 1000 µg/L.9 In Allen et al, the median ferritin level on presentation in children with HLH was 5992 µg/L, and the median maximum ferritin level was 15 830 µg/L, suggesting a higher ferritin threshold would be more specific for HLH.
Markedly elevated serum ferritin is typically thought to occur only in a few conditions, including AOSD, MAS, and the related HLH. In the pediatric setting, highly elevated levels have been found to be specific for HLH. The retrospective analysis performed by Allen et al at Texas Children’s Hospital reported that maximum ferritin level higher than 10 000 μg/L had a 90% sensitivity and 98% specificity for HLH.8 In contrast, our results in the adult population suggest that a serum ferritin elevation more than 5 times this level is associated with a variety of disorders and is not predictive of HLH.
In our adult cohort, ferritin levels higher than 50 000 μg/L were seen most often in patients with renal failure, hepatocellular injury, infections, and hematologic malignancies. Nineteen percent of patients had HLH/MAS. Of the patients with HLH/MAS, 53% also developed significant hepatocellular injury and 53% developed renal failure, including 32% with both. The congruence of hepatic and renal failure in these patients is not surprising, given the generally fulminant and severe nature of HLH, leading many patients to be profoundly ill in the intensive care unit. In addition, liver dysfunction has become a well-recognized consequence of HLH.4 There was no ferritin value above which ferritin was specific for HLH/MAS. This is in stark contrast to what is seen in the pediatric population and suggests that highly elevated ferritin is not specific for HLH/MAS in adults.
Much of our understanding of HLH is based on the pediatric population and experience with children, most of whom have familial HLH. This is frequently extrapolated to the adult setting. Although many of the clinical and laboratory manifestations of HLH appear to be similar in these 2 populations, one must exercise caution when generalizing from the understanding of pediatric disease. Children with HLH are more likely than adults to have familial HLH caused by an inherited defect in immune regulation. In addition, the spectrum of diseases seen in the pediatric population is different than in the adult population. Prospective studies are necessary to identify and verify which criteria are most useful for diagnosing HLH in adults. When comparing our results to the adult cohort in Moore et al, a larger proportion of patients have HLH/MAS when using the higher ferritin cutoff value of 50 000 μg/L as opposed to 1000 µg/L (19% vs <1%). Although the higher ferritin threshold increases the probability that a patient identified will have HLH, the majority of adults with this degree of ferritin elevation do not have HLH. A recent study by Fardet et al reported that the fraction of glycosylated ferritin is lower in patients with HLH compared with other inflammatory disorders.30 Further investigation is necessary to validate this observation and identify novel markers of HLH.
Our multivariable analysis revealed that hemolytic anemia was the only variable independently associated with a differential ferritin level. Although hemolytic anemia was only present in a minority of the patients, those patients had significantly higher ferritin levels compared with patients without hemolytic anemia.
In a separate analysis of 50 patients with HLH at BWH and MGH, the median ferritin level on presentation was 5823 µg/L (minimum, 461 µg/L; maximum, 98 110 µg/L), and the median maximum ferritin level was 19 687 µg/L (minimum, 1618 µg/L; maximum, 202 911 µg/L). Only 10 of 50 patients had a maximum ferritin level higher than 50 000 μg/L, suggesting that the high ferritin cutoff of 50 000 μg/L is not sensitive for HLH (A.M.S., A.M., and N.B., unpublished data). Notably, the current diagnostic criteria for HLH used a ferritin cutoff of 500 μg/L. All but 1 of the identified patients with HLH had admission ferritin levels greater than this value, and all patients had maximum ferritin levels higher than 500 μg/L, so the negative predictive value of a normal ferritin is high. We advocate checking ferritin levels in every patient with suspected HLH. If the ferritin level is lower than 500 μg/L, the patient is unlikely to have this disorder. Although highly elevated ferritin levels can be seen in HLH, a value higher than 50 000 μg/L is not diagnostic, and additional work-up is necessary before confirming that the patient has HLH. In the same analysis of 50 patients with HLH, 30/33 patients checked for sIL2-R had levels of 2400 U/mL or higher. Similarly, our current study shows that 10/10 patients with HLH evaluated for sIL2-R had elevated levels. Larger studies in adults with and without HLH are necessary to determine the sensitivity and specificity of elevated sIL2-R in adults with HLH.
Inherent in the retrospective design of this study are limitations. Although we can identify disorders that occur with highly elevated ferritin, we cannot infer causality. In addition, we decided to record all conditions present in each patient, rather than choose a single disorder presumed to be the cause of elevated ferritin. This approach decreases the chance that our own biases will skew the results, but risk of confounding remains. For example, the high prevalence of renal failure in our cohort may not reflect the fact that renal failure is the most common cause of elevated ferritin but, instead, reflect that renal failure frequently occurs in the setting of other illnesses, including infection, liver failure, and malignancy. In addition, our study required manual review of the charts, limiting the information obtainable to only what was documented in the electronic record. Last, this study was carried out at a tertiary care center affiliated with a comprehensive cancer center. The patient population may not represent that found in general practice.
Hemophagocytic lymphohistiocytosis is a frequently fatal disorder that is currently diagnosed according to a constellation of clinical and laboratory abnormalities. Highly elevated serum ferritin levels are reported to be specific for HLH in the pediatric setting. Our results suggest that this is not the case for adults and that marked hyperferritinemia can be seen in a variety of conditions. Further research is required to find a more reliable marker for diagnosing HLH in adults.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M.S., the primary author of the manuscript, contributed to the study’s conception, design, and analysis, and the interpretation of the data; F.C. and D.N. helped with statistical analysis and data interpretation; A.M. contributed to the study’s conception and helped direct the project; E.M. contributed to the study conception; N.B., the principal investigator, contributed to the study’s conception and design and the data interpretation; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nancy Berliner, Brigham and Women's Hospital, Division of Hematology, 75 Francis St, Mid-Campus 3, Boston, MA 02115; e-mail: nberliner@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal