In this issue of Blood, Gröschel et al and Lavallée et al report on a subset of myeloid malignancies with inv(3)(q21q26)/t(3;3)(q21;q26).1,2 Both groups demonstrate concordantly that this subset exhibits a distinct gene expression profile, a high rate of mutations activating the RAS/receptor tyrosine kinase (RTK) pathways, and frequent mutations in genes coding for splice factors, epigenetic modifiers, and transcription factors.1,2
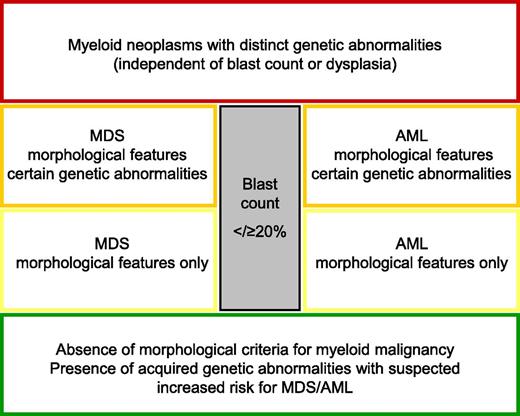
Proposal for a “work-in-progress concept” to classify myeloid malignancies until all questions are answered by genetic information.
Proposal for a “work-in-progress concept” to classify myeloid malignancies until all questions are answered by genetic information.
Further, Lavallée et al detected mutations in IKZF1 only in ecotropic viral integration-1 (EVI1)-rearranged acute myeloid leukemia (AML) but not in any of 139 AMLs without EVI1 rearrangement.2 In agreement with previous work in which Gröschel et al had shown that EVI1 becomes activated via structural repositioning of a distal GATA2 enhancer from 3q21 to the EVI1 locus at 3q26,3 they suggest to update the currently annotated World Health Organization (WHO) AML subtype AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2); RPN1-EVI1 into AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2); GATA2-EVI1. Further, cases are suggested to be classified into this category irrespective of blast counts, similar to WHO AML categories t(8;21); RUNX1-RUNX1T1, inv(16)(p13q22)/t(16;16)(p13;q22);CBFB-MYH11 or t(15;17)(q22;q12); PML-RARA,4 as Gröschel et al were not able to demonstrate any differences between myelodysplastic syndrome (MDS) and AML with inv(3)(q21q26)/t(3;3)(q21;q26).1
This suggestion can be taken as an opportunity to reevaluate the classification of myeloid malignancies in general and specifically the arbitrary distinction between MDS and AML based only on percentage of blast counts. Drawing the border between MDS and AML has been under debate for many years. In the era of the French-American-British classification, the threshold between MDS and AML was first determined by blast count. A myeloid neoplasms with 21% to 30% of blasts in the bone marrow was classified as MDS RAEB-t (myelodysplastic syndrome, refractory anemia with excess of blasts in transformation), whereas myeloid malignancies with >30% of blasts in the bone marrow were called AML.5 The WHO classification in 2001 lowered the border between MDS and AML to 20% of bone marrow blasts.6 This decision was mainly based on clinical data demonstrating that clinical outcome between MDS RAEB-t and AML is comparable and that patients with MDS RAEB-t seem to benefit from “AML” therapy.7
The data presented in Blood support the notion that the time has come to change concepts substantially. This change of concept was already started with the WHO classification in 2008 by classifying a disease according to distinct genetic abnormalities irrespective of blast count as for t(8;21)(q22;q22); RUNX1-RUNX1T1, inv(16)(p13q22) or t(16;16)(p13;q22); CBFB-MYH11, and t(15;17)(q22;q12); PML-RARA and needs to be further developed. A further candidate is suggested by Gröschel et al and supported by Lavallée et al: inv(3)(q21q26.2) or t(3;3)(q21;q26.2); GATA2-EVI1. Other myeloid malignancies with recurrent rearrangements leading to gene fusions such as MLL rearrangements or t(6;9)(p23;q34)/DEK-NUP214 and t(1;22)(p13;q13)/RBM15-MKL1 were already introduced into the WHO classification as distinct entities. Further, the following subsets, which have been shown to fulfill the criteria of entities such as t(8;16)(p11;p13)/MYST3-CREBBP, t(3;5)(q25;q35)/NPM1-MLF1, t(7;11)(p15;p15)/NUP98-HOXA9, t(16;21)(p11;q22)/FUS-ERG, and t(5;11)(q35;p15)/NUP98-NSD1, could be considered. In a revised classification, these entities might all be called “myeloid neoplasms with a distinct genetic abnormality,” irrespective of blast counts. The remaining AML without a yet elucidated defining genetic background might be further separated into MDS and AML until the identification of a distinct underlying genetic event (see figure).
A second issue becomes obvious: the concept of primary and secondary genetic abnormalities, suggesting there is a clear chronological order that needs to be challenged. The data of Gröschel et al support the current observations that various routes of mutation acquisition are possible, although certain chronological orders are more likely than others.1 Clonality analyses revealed that, in the majority of inv(3)/t(3;3) carrying AML and MDS cases, RAS and RTK mutations, as well as the other mutations, were present together in the main clone. However, in 3 AML cases, the 3q rearrangement was found in the major clone, whereas the RAS pathway mutations were only present in a subset of these cells. In contrast, in 2 other cases, the inv(3)/t(3;3) aberrations were present only in a subclone of cells with other mutations. In chronic myeloid leukemia, it is well established that the BCR-ABL1 rearrangement is the primary event and thus occurs before additional rearrangements such as 3q abnormalities, which are typically acquired in transition to blast crisis. However, rare cases with AML were identified in which the BCR-ABL1 rearrangement was a secondary event to a variety of different primary abnormalities such as inv(16), t(8;21), 5q deletion, or NPM1 mutation.8 Thus, current knowledge supports the view that any alteration can occur first and may be complemented by any other genetic abnormality. The first hits define the disease and the likelihood of certain further genetic alterations that promote the disease.
Although a major step is to define disease entities based on genetic events, an even more important aspect will be to discriminate between normal hematopoiesis and hematological disease. The tremendous increase in the knowledge of genetic imbalances and mutations has led to an increase of genetic analyses in patients with suspected diseases and even in the normal population. Frequently, the detection of a genetic abnormality is taken as proof that a clonal disease is present. However, Jacobs et al observed mosaic abnormalities, either aneuploidy or copy-neutral loss of heterozygosity, of >2 Mb in size in autosomes of cancer-free individuals. This frequency increased with age, from 0.23% at <50 years to 1.91% between 75 and 79 years.9 In line with this, Laurie et al found that detectable clonal mosaicism in peripheral blood is low (<0.5%) from birth until 50 years of age and rises rapidly to 2% to 3% in the elderly. Many of the genetic alterations observed were characteristic of those found in hematological cancers. Although only 3% of subjects with detectable clonal aberrations had any record of hematological cancer before DNA sampling, those without a previous diagnosis had an estimated 10-fold higher risk of a subsequent hematological cancer.10
In summary, Gröschel et al and Lavallée et al provide important and novel insights into the biology of myeloid neoplasms with inv(3)(q21q26.2) or t(3;3)(q21;q26.2); GATA2-EVI1 rearrangement.1,2 Further, and possibly even more important, they initiate a discussion on novel concepts of classification and the definition of diseases against the background of current genetic knowledge.
Conflict-of-interest disclosure: C.H. has part ownership in the MLL Munich Leukemia Laboratory.