In this issue of Blood, Scheicher et al show that cyclin-dependent kinase 6 (CDK6) has a novel role in the regulation of both normal and leukemic stem cells. It acts as a transcriptional suppressor of Egr1, enabling it to control both normal and leukemic stem cell activation.1
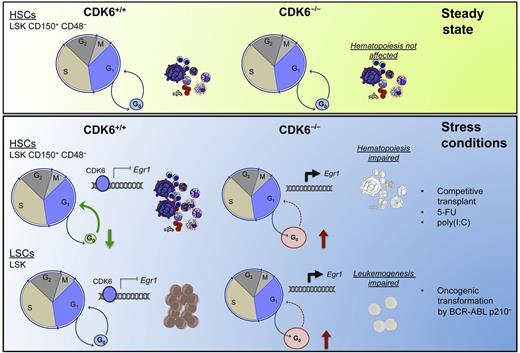
CDK6 loss in steady-state conditions does not significantly affect the distribution of LSK populations (top panel). Stress conditions will result in a rapid exit from G0 of HSCs as a result of a CDK6-dependent transcriptional repression of Egr1. Loss of CDK6 prevents the repression of Egr1 to allow cells to exit G0. In leukemia models in which CDK6 is aberrantly activated, the exit of LSCs from the G0 phase depends on CDK6. CDK6 loss results in failure of the leukemogenesis process (bottom panel). 5-FU, 5-fluorouracil; poly(I:C), polyinosinic:polycytidylic acid.
CDK6 loss in steady-state conditions does not significantly affect the distribution of LSK populations (top panel). Stress conditions will result in a rapid exit from G0 of HSCs as a result of a CDK6-dependent transcriptional repression of Egr1. Loss of CDK6 prevents the repression of Egr1 to allow cells to exit G0. In leukemia models in which CDK6 is aberrantly activated, the exit of LSCs from the G0 phase depends on CDK6. CDK6 loss results in failure of the leukemogenesis process (bottom panel). 5-FU, 5-fluorouracil; poly(I:C), polyinosinic:polycytidylic acid.
Mammalian CDKs are widely recognized for their well-established role in orchestrating the steps of cell-cycle progression.2 CDK4 and CDK6 form a complex with cyclin D to promote the G1 to S phase progression through the phosphorylation of retinoblastoma (Rb) protein and transcription factors with roles in proliferation and differentiation. CDK4 and CDK6 have been found to be aberrantly regulated in many tumor types, including mixed lineage leukemia (MLL)–rearranged leukemia,2,3 positioning potential CDK4/6 as therapeutic targets.4 Loss of CDK6 produces defects in hematopoietic cell proliferation and minor anemia.5 Quiescent hematopoietic stem cells (HSCs) can be activated to enter the cell cycle by stress conditions.6 These observations suggest that CDK6 is required for expansion of certain differentiated compartments rather than for proliferation of early hematopoietic precursors. Like HSCs, leukemia stem cells (LSCs) are quiescent and enter the cell cycle as required to repopulate the leukemia, a property thought to confer resistance to chemotherapy.7 Because of the critical role of HSCs and LSCs in providing a reservoir for hematopoiesis and leukemogenesis, respectively, Scheicher et al investigated the role of CDK6 in HSCs and LSCs.
The authors found that CDK6−/− HSCs are unable to compete effectively with CDK6+/+ HSCs in repopulating the hematopoietic lineages in lethally irradiated mice despite the presence of CDK6−/− LSK (Lin−, Sca+, c-Kit+) cells in the bone marrow of the transplanted animals. Importantly, these cells did not show homing defects. Interestingly, under steady-state conditions, CDK6−/− HSCs did not exhibit any significant changes in the distribution of LSK populations, consistent with previous reports.8 However, when further fractionating the populations, the authors found an increase in the quiescent LSK fraction (LSK, CD150+, CD48−, CD34−, CD135−) when compared with the multipotent progenitor fraction (LSK, CD150+, CD48−, CD34+, CD135−). No differences were found in the cell-cycle status of LSK populations between CDK6−/− and CDK6+/+. In contrast, under stress conditions (such as treatment with 5-fluorouracil), CDK6 was found to be required for dormant HSCs to exit G0. Furthermore, the authors showed that the CDK6−/− LSK, CD150+, CD48− population could not be forced to a rapid entry to the cell cycle using polyinosinic:polycytidylic acid.
Next, the group investigated how CDK6 controls the exit from quiescence of HSCs. Marked downregulation of Egr1 occurred upon polyinosinic:polycytidylic acid treatment in the CDK6+/+ LSK, CD150+, CD48− population when compared with the CDK6−/− LSK, CD150+, CD48− cells, suggesting that CDK6 directly controls Egr1, a transcription factor known to regulate HSC quiescence.9 The authors found that CDK6 is able to bind the Egr1 promoter in a kinase-independent manner, using the kinase-inactive mutant form of CDK6 (CDKK43N). Further transcriptional network analyses corroborated that the CDK6-Egr1 axis is important for the control of exit from quiescence of HSCs.
Because quiescent LSCs play a crucial role in the evasion of therapy, the authors then tested whether CDK6 also controlled the quiescence of malignant stem cells using BCR-ABLp210+–induced leukemia. Indeed, CDK6 was necessary for maintaining the leukemia because all CDK6+/+ mice succumbed to the disease. In contrast, the absence of CDK6 resulted in decreased colony formation and increased Egr1 expression in LSK p210+ populations. Importantly, knockdown of Egr1 using short hairpin RNA rescued the leukemic colony formation of the CDK6−/− BCR-ABLp210+ cells.
The novel role of CDK6 in the regulation of quiescence under stress conditions (including oncogenic transformation) suggests that CDK6 represents a potential target to prevent quiescent LSCs from propagating disease without affecting normal HSCs (in the steady state) (see figure). The data invite further investigation to understand the cell cycle– and kinase-independent transcriptional regulatory roles unique to CDK6 to develop novel inhibitors specific to this function of CDK6 to facilitate selective targeting of malignant LSCs without affecting normal steady-state hematopoiesis.
Conflict-of-interest disclosure: The author declares no competing financial interests.