In this issue of Blood, Kolstad et al report an elegantly designed and well-implemented study showing that intratumoral injection of ex vivo-produced immature dendritic cells (DCs), granulocyte macrophage colony-stimulating factor (GM-CSF), and low-dose rituximab into an irradiated tumor elicits objective responses at untreated sites and that these clinical remissions correlate with the induction of tumor-reactive CD8 T-cell responses.1
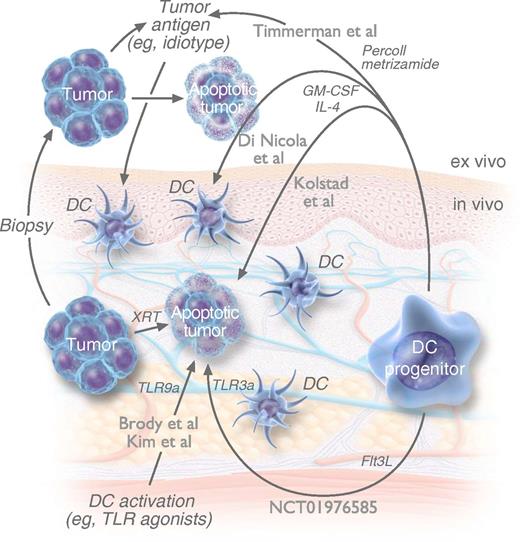
These similar approaches of bringing together DCs and tumor antigens have been tested clinically. Each approach differs in the site and technique of isolating, loading, and activating DCs, by either ex vivo Percoll and metrizamide gradient separation with ex vivo Id pulsing4 ; ex vivo monocyte culture in GM-CSF/IL-4 with ex vivo apoptotic tumor pulsing5 ; in vivo (scarce) DCs and (abundant) tumor B-cell TLR activation with in vivo tumor irradiation7 ; ex vivo monocyte culture in GM-CSF/IL-4 with in vivo tumor irradiation, GM-CSF, and rituximab1 ; or in vivo Flt3L-mobilized DCs with in vivo tumor irradiation and TLR activation. NCT01976585 refers to the trial registry number (clinicaltrials.gov) of a clinical trial currently in progress. Flt3L, FMS-related tyrosine kinase 3 ligand. Professional illustration by Luk Cox, Somersault 18:24.
These similar approaches of bringing together DCs and tumor antigens have been tested clinically. Each approach differs in the site and technique of isolating, loading, and activating DCs, by either ex vivo Percoll and metrizamide gradient separation with ex vivo Id pulsing4 ; ex vivo monocyte culture in GM-CSF/IL-4 with ex vivo apoptotic tumor pulsing5 ; in vivo (scarce) DCs and (abundant) tumor B-cell TLR activation with in vivo tumor irradiation7 ; ex vivo monocyte culture in GM-CSF/IL-4 with in vivo tumor irradiation, GM-CSF, and rituximab1 ; or in vivo Flt3L-mobilized DCs with in vivo tumor irradiation and TLR activation. NCT01976585 refers to the trial registry number (clinicaltrials.gov) of a clinical trial currently in progress. Flt3L, FMS-related tyrosine kinase 3 ligand. Professional illustration by Luk Cox, Somersault 18:24.
In 1973, Ralph Steinman and Zanvil Cohn at Rockefeller University described a novel and rare leukocyte subset in lymphoid organs that “constantly puts out and retracts small cytoplasmic dendrites.”2 The unique morphology of these DCs was gradually shown to underlie a unique function: a quantitatively and qualitatively enhanced capacity for presenting antigens to T cells. Studies of these professional antigen-presenting cells (APCs) progressed from in vitro to in vivo, from mice to humans, and from preclinical experiments to clinical trials showing that antigen-pulsed DC vaccination induces antigen-specific T cells in a setting in which antigen alone does not.3 Effective preclinical antipathogen DC vaccines motivated the development of anticancer DC vaccines in numerous histologies, including melanoma, prostate, and kidney cancer, some of which yield immune responses and even survival benefit, although DC-based vaccines for lymphoma have particularly demonstrated objective clinical responses, even when used as monotherapy.1,4-7 Although these small studies similarly reproduce the anticancer effect of DC-based lymphoma vaccines, the differences—and the hopes for greater future success—are in the details. Each approach aims to ultimately present tumor-associated antigens on a sufficient number of activated DCs, although with differing methods for (1) DC production, (2) tumor antigen loading, and (3) DC activation (see figure). Each of these steps can be accomplished ex vivo or in vivo: the latter with pragmatic advantages, and the former with greater opportunities to assess and control each step.
In 2002, investigators from Stanford published final results of a study using ex vivo idiotype (Id)-pulsed, Percoll, and metrizamide gradient-purified DCs in 35 lymphoma patients, demonstrating induction of T-cell proliferative responses to Id protein, as well as complete and molecular remissions.4 Although most patients mounted anti-Id T-cell responses, there was no clear correlation between T-cell response and clinical benefit. In 2009, investigators from Milan attempted to both obviate the need for antigen identification/purification and broaden the spectrum of target tumor-associated antigens by using ex vivo monocyte-derived (GM-CSF- and interleukin [IL]-4-treated) DCs pulsed with autologous, apoptotic (heat-shock, γ-irradiated, and ultraviolet C-treated) tumor cells. There were 6 partial and complete responses, and these correlated with reductions in peripheral blood regulatory T cells (Tregs) and increases in natural killer cells, although there was no clear relationship between the induction of tumor-specific T-cell responses and clinical benefit.5 Two subsequent studies from Stanford sought to circumvent the need for ex vivo DC production/antigen exposure by using low-dose radiotherapy to load antigens from dying tumor cells onto the scarce peritumoral DCs followed by intratumoral injections of a Toll-like receptor 9 (TLR9) agonist to activate both these antigen-loaded DCs and possibly residual malignant B cells themselves to function as amateur APCs. These studies demonstrated partial and complete clinical responses, the correlation of in vitro tumor induction of Tregs with worse clinical response, and the potential to achieve more rapid and greater magnitude responses with repeated booster vaccination.6,7
Kolstad et al from Oslo University Hospital show the utility of using ex vivo-produced immature monocyte-derived DCs (similar to the Milan approach) but then facilitating tumor antigen uptake and activation of these DCs in vivo at an irradiated tumor site (similar to the recent Stanford approach), along with GM-CSF and low-dose rituximab, to enhance Fc receptor-mediated phagocytosis. The present study assessed clinical response by standard (International Working Group) criteria excluding the irradiated site and assessed tumor-specific CD4 and CD8 T-cell responses using a straightforward flow cytometry-based proliferation assay after 5-day coculture with the autologous tumor. The authors demonstrate a significant proportion of partial and complete responses (36% of all patients) and—perhaps most significantly—a statistically significant correlation of tumor-specific CD8 T-cell responses with clinical response, in some distinction to the aforementioned studies. The successful correlation of immunologic and clinical responses—if reproducible—represents a major step in accelerating the future of the field, creating opportunity for the use of immune response as a rapidly assessable surrogate end point (eg, comparing multiple iterations of the vaccine maneuver); assessment of vaccine efficacy when clinical end points would be confounded (eg, when used in combination or adjuvant settings); and analysis of mechanism (eg, probing the flow cytometrically defined tumor-reactive T cells for clonality [by high-throughput TRBV sequencing], reactivity to patient-specific genomically determined neoantigens, and expression of costimulatory or checkpoint molecules to suggest rational combination therapies).
The pragmatic consideration of in vivo DC loading demonstrated in this study is also a meaningful advance in the field as it reduces resource intense ex vivo processing from 2 individualized products (in the Stanford DC-Id and Milan DC-apoptotic tumor studies) to 1 individualized product (immature DCs). To further simplify the bringing together of large numbers of DCs with tumor antigens, our group developed a novel iteration of the approach that produces, loads, and activates DCs entirely in vivo. This study (#NCT01976585) uses intratumorally administered Flt3L to increase intratumoral DCs, followed by low-dose radiotherapy and an intratumorally administered TLR3 agonist, to generate tumor antigen-loaded activated DCs with no ex vivo processing. Preliminary results demonstrated partial and complete responses of patients with bulky and leukemic-phase low-grade lymphoma. A similar approach using Flt3L and a DC-targeting antibody-tumor antigen conjugate is being assessed by the Cancer Immunotherapy Trials Network for patients with advanced stage melanoma (#NCT02129075).
Bringing DCs and tumor antigens together is achievable and—as confirmed now in technically distinct but conceptually similar studies—can induce objective remissions in patients with low-grade lymphoma with a remarkable safety profile. The commendable study by Kolstad et al both fortifies and advances progress in this field. Their approach is both effective in its current form and extremely promising due to its potential for further optimization. Together, these DC vaccine studies improve our understanding of assessing meaningful antitumor immune responses and improve our outlook for safe and effective antitumor immunotherapy for our patients.
Conflict-of-interest disclosure: J.D.B. receives research funding from the Celldex Therapeutics. The remaining author declares no competing financial interests.