Abstract
Histologic transformation of follicular lymphoma to an aggressive non-Hodgkin lymphoma is a critical biologic event with profound implications on the natural history of this otherwise indolent disease. Recent insights into the genetic and epigenetic basis of transformation have been described, with the recognition of pivotal events governing the initiation and persistence of tumor evolution. Outcomes of patients with transformed lymphoma have historically been poor; however, several studies in the rituximab era suggest that survival may be more favorable than previously recognized. This review highlights our current understanding of transformed follicular lymphoma biology and pathogenesis, current treatment, and future directions.
Introduction
Histologic transformation (HT) refers to a biologic event leading to the development of a high-grade, aggressive non-Hodgkin lymphoma in patients with an underlying follicular lymphoma (FL).1 Transformation to diffuse large B-cell lymphoma (DLBCL) or Burkitt lymphoma is also known to take place in other subtypes of indolent lymphoma such as marginal zone lymphoma, lymphoplasmacytic lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, and lymphocyte predominant Hodgkin lymphoma, but is best described as occurring in FL.2 For the purposes of this review, we will focus our discussion on histologic transformation of FL specifically to DLBCL.
Biology of transformation
Origin of HT
Although useful for clinical care, the term “high-grade transformation” may obscure the biology of FL for 2 reasons. First, the natural history of FL from its beginning is likely a series of transformations converting a FL-precursor cell to incrementally greater malignant potential or varying its clinical behavior. Whereas the characteristic IGH:BCL2 translocation arises in early B cells within the marrow environment, these cells are common in healthy individuals. The subsequent accumulation of oncogenic mutations sufficient to produce bona fide FL is thought to occur through the mistargeted activity of activation-induced deaminase (AID) which is expressed in the precursor-FL cells as they iteratively reside in germinal centers (GCs) in lymph nodes. Expression of AID in normal GCs results in somatic hypermutation (SHM) of immunoglobulin loci. This collateral damage that is done to the genome, termed aberrant SHM (aSHM), may be at the root of the many B-cell malignancies that have the follicular B cell as the cell of origin. The second reason that the term “transformation” may cause misconceptions is that only rarely does the DLBCL “transform” directly from the most abundant cell population present in the FL at the time of diagnosis. Arguably every study that has examined serial specimens using genetic methods has found examples of “nonlinear” transformation in which the clone detected at transformation is more closely related to a common progenitor than the clone predominating at the time (or site) of prior sampling.3-11 Newer studies with greater detail of genetic analysis reveal a higher fraction of the patients with nonlinear transformation, and it seems likely that nonlinear transformation is the rule rather than the exception. Most recent whole-exome studies show definitively that the low-grade FL at diagnosis frequently possesses one or more “driver” mutations not found in the subsequent transformed FL.3,4,9 Although the term “high-grade transformation” implies a binary switch from indolent to aggressive disease, the reality is that each FL is probably undergoing multiple “transformations” during the life of any patient with this low-grade disease. This observation indicates that the FL tumor cell population is composed of numerous subpopulations with distinct driver mutations and presumably distinct sensitivities to therapies, particularly targeted ones. Sequencing results obtained on any 1 biopsy from a patient is only an incomplete picture of the tumor’s genetic composition and thus an inaccurate picture of the responsiveness to targeted therapies. Recognizing this model of continuous tumor evolution may allow investigators to target the mechanisms causing continued genetic instability (such as inhibiting AID), thus potentially interdicting the progression of FL.
Drivers of HT
Detailed genetic analyses indicate there is no single mechanism driving transformation from FL to DLBCL.3-5,12 Rather, studies suggest several discrete mechanisms are likely affected, including alterations of cell-cycle control (through mutation or deletion of cyclin-dependent kinase 2A/B [CDKN2A/B] and alterations in myc) and impairment of the DNA damage response (through loss of p53 and/or CDKN2A).3,4 Furthermore, there are consistent losses of genes associated with regulation of the immune response, such as the entire HLA class 1 locus, mutations specifically in β-2-microglobulin (B2M; a critical component of the class 1 complex), and mutations in CD58 (involved in regulating the complement-mediated effects on cells).4,5,13 In general, the driver mutation profile of transformed FL resembles that of GC B (GCB) DLBCL and may lack the mutations in the B-cell receptor (BCR) signaling pathway that are characteristic of activated B-cell (ABC) DLBCL (such as CD79b).
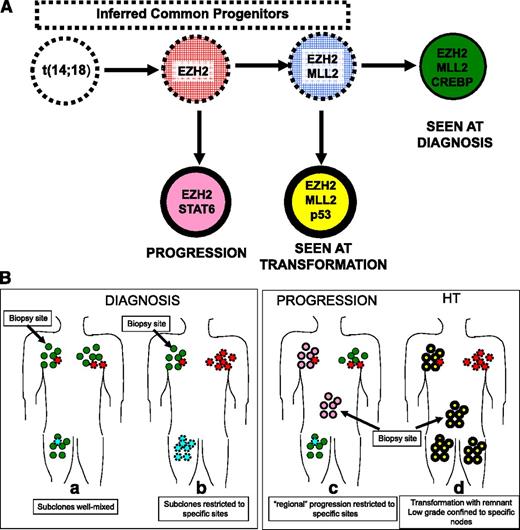
In addition to enumerating the specific point mutations in coding regions that may drive transformation, the studies of paired low-grade and HT specimens have also described qualitative changes in genetic features, which suggest the mechanisms permitting these mutations to occur in the first place. Changes in chromosomal copy number (“chromosomal number variation” [CNV]) have been implicated in HT, yet it remains unclear if the acquisition of CNV is a major genetic mechanism driving transformation. Eide et al found no evidence that globally increased CNV number predicted transformation,8 whereas Pasqualucci et al found that a statistically significant (although modest) increase in genomic complexity accompanied transformation based on CNV analysis.4 Recent efforts to identify mutations associated with HT have also defined which mutational events are early in the life history of FL (Figure 1Ba-b). These early lesions in FL primarily affect epigenetic regulators (genes controlling chromatin structure), including MLL2 (more formally known as KMTD), EZH2, and CREBBP.3,4,14,15 Pasqualucci et al suggest that these mutations may abet the “mutator” phenotype of the FL, allowing AID to access inappropriate regions of the genome, leading to aSHM, and genetic instability driving further transformation. These data raise the possibility that epigenetic dysregulation may be key to development of HT, but perhaps not critical to the aggressive phenotype once established. Furthermore, the observation by Pasqualucci that HT is associated with a striking increase in aSHM4 implies that recognition of intraclonal variation due to aSHM may be a predictor of HT.
How coupling data from mutation analyses and the complex clonal architecture yields insights in the clinical behavior of FL. (A) The complex clonal architecture of follicular lymphoma. (B) (a) The earliest step in the development of FL is acquisition of the IGH:BCL2 translocation. Cells carrying this lesion are frequently found in healthy individuals. Subsequent but still early steps in development of FL include mutations that disrupt regulation of chromatin structure, including mutations in EZH2 and MLL2. In this hypothetical patient, cells carrying just these mutations were not detected (red and blue cells with dashed borders) but their existence can be inferred from the detection of various cell populations carrying these same mutations as well as additional mutations (green, rose, and yellow cells with solid borders). Later events that are associated with HT include loss (or mutation) of TP53 and CDKN2A (yellow). It appears that in perhaps all cases that are studied with sufficiently sensitive techniques, the population that arises at HT (yellow) is not directly descended from the population detected at the time of diagnosis (green) or at relapse (rose). (b) The complex clonal architecture of FL raises 2 possible scenarios for the distribution of subclones at the time of diagnosis. Either the subclones are well mixed (a) or the subclones are relatively restricted to specific sites (b). For example, there is observational evidence that subclones in the marrow and the nodes are distinct and show only limited mixing in FL. At progression and transformation (c-d), the inhomogeneous distribution of subclones requires that selection of the biopsy site is based on clinical features or PET scan, so that the site of the aggressive subclone is sampled rather than a site composed of a low-grade subclone.
How coupling data from mutation analyses and the complex clonal architecture yields insights in the clinical behavior of FL. (A) The complex clonal architecture of follicular lymphoma. (B) (a) The earliest step in the development of FL is acquisition of the IGH:BCL2 translocation. Cells carrying this lesion are frequently found in healthy individuals. Subsequent but still early steps in development of FL include mutations that disrupt regulation of chromatin structure, including mutations in EZH2 and MLL2. In this hypothetical patient, cells carrying just these mutations were not detected (red and blue cells with dashed borders) but their existence can be inferred from the detection of various cell populations carrying these same mutations as well as additional mutations (green, rose, and yellow cells with solid borders). Later events that are associated with HT include loss (or mutation) of TP53 and CDKN2A (yellow). It appears that in perhaps all cases that are studied with sufficiently sensitive techniques, the population that arises at HT (yellow) is not directly descended from the population detected at the time of diagnosis (green) or at relapse (rose). (b) The complex clonal architecture of FL raises 2 possible scenarios for the distribution of subclones at the time of diagnosis. Either the subclones are well mixed (a) or the subclones are relatively restricted to specific sites (b). For example, there is observational evidence that subclones in the marrow and the nodes are distinct and show only limited mixing in FL. At progression and transformation (c-d), the inhomogeneous distribution of subclones requires that selection of the biopsy site is based on clinical features or PET scan, so that the site of the aggressive subclone is sampled rather than a site composed of a low-grade subclone.
Predictors of risk
In efforts to identify the mechanisms driving transformation, several groups have looked at possible biologic and molecular features as predictors of HT. Gene expression profiling (GEP) studies have identified features of the immune microenvironment that predict progression or transformation.16-19 Two general models explaining the tumor microenvironment’s support of lymphomagenesis have been widely discussed: either the microenvironment supports proliferation (or blocks apoptosis) directly, or it offsets intrinsic antitumor immunologic effects.20,21 Existing data support both of these hypotheses although the studies of the exact mechanisms remain contradictory.20 Genetic studies provide a possible alternative understanding for the relationship between the immune microenvironment and HT. For example, the loss of class 1 and B2M expression (features now shown to be associated with HT) would be expected to result in an alteration in the immune components of the microenvironment if it preceded overt HT. Furthermore, the immune microenvironment may affect AID expression in FL cells; because AID is possibly at the root of the “mutator” phenotype, facilitating evolution of FL, the immune microenvironment may directly regulate the emergence of an aggressive subclone. Just as mutations found in the class 1 or B2M might be at the root of the GEPs that suggest a role for the immune microenvironment in determining transformation, perhaps the changes in GEP due to epigenetic dysregulation result in the “pluripotency” (or stem-cell like) gene expression pattern that Gentles et al correlated with a propensity to transform.22 Similarly, the observation that a small gene expression panel based on components of the nuclear factor κB (NF-κB) pathway components is predictive of HT may directly reflect the recent sequencing data showing that HT is frequently accompanied by mutations in genes encoding components of the NF-κB pathway, as mutations affecting the NF-κB pathway appear to be among the most consistent of the stepwise changes that ultimately lead to clinically recognizable HT.3,23
Summary: biology of transformation
The recent genetic analyses of paired FL/HT specimens potentially link several disparate parameters that have been shown to predict HT, and have emphasized the complex clonal architecture of FL, a complexity which is consistent with the clinical behavior. These genetic data suggest attractive therapeutic targets, thought to be those mutations which occur earliest, such that intervening on these would affect both the DLBCL and the numerous underlying FL precursor clones, and thus potentially reducing the likelihood of relapse.
Clinical definition of transformation
When determined pathologically through a biopsy, transformation is defined by the histologic documentation of increased numbers of large cells that eradicate the follicular architecture, leading to a high-grade lymphoma that is related to the original FL.24 Establishing HT with a biopsy is considered the gold standard diagnostically, and whenever possible, suspicion of HT should be verified pathologically. The resultant DLBCL frequently maintains a GC pattern, but with large cells infiltrating the lymph nodes diffusely and leading to effacement of the follicular structure. In the absence of a tissue diagnosis, HT has been reliably established clinically in the presence of rising lactate dehydrogenase (LDH), rapid nodal growth, sudden decline in performance status, new “B” symptoms or hypercalcemia, or new involvement in extranodal sites of disease.25-27 Studies using clinical criterion to establish transformation have found reliably similar rates of survival compared with patients in whom HT was based on a biopsy. To further assist in the diagnosis of HT, 18[F] fluorodeoxyglucose (FDG) positron emission tomography (PET) scans have also become useful tools in predicting HT, with higher standardized uptake values (SUVs) correlating with more aggressive histology.28,29 SUVs above 10 in 1 study were able to reliably predict aggressive lymphoma with a specificity of 80%; values above 13 did so with 90% certainty.30,31 In a study focusing on PET in defining HT, the SUVs in biopsy-proven sites of transformation ranged from 3 to 38, with a mean of 14 and median of 12.29 Bodet-Milin and colleagues recapitulated these findings in a study of 38 patients with clinically suspected transformation of indolent lymphoma.32 HT was detected pathologically in 45% of cases (17 of 38). Among patients with SUVmax of >17, the positive predictive value of FDG-PET for detecting HT was 100%. On the contrary, SUV < 11.7 was associated with a low risk of HT, such that no patient with SUVmax < 11.7 showed HT. Given the overlap in the degree of FDG uptake between indolent and aggressive lymphomas and the lack of data supporting a specific practice, FDG-PET is not likely to replace biopsy as the gold standard to confirm HT. However, in very select circumstances, such as in the setting of an SUV above 17, in a patient with an inaccessible biopsy site, rapidly deteriorating clinical status, and other clinical features suggestive of HT, it may be reasonable to consider foregoing a biopsy and therapeutically approach the disease as transformed histology.
Incidence of HT
Estimates of the true incidence of HT in FL have wavered over the past several decades. Most studies assessing HT did not determine clonality of paired specimens, in order to determine true HT events from de novo aggressive lymphomas, and are based on retrospective series. Many of the publications addressing the frequency of HT occurred prior to the use of rituximab as treatment of lymphoma and, using both clinical and pathologic criteria, have reported broad estimates of risk ranging from 24% to 70% overall,27,33 11% to 17% at 5 years,25,27 and ∼30% at 10 years.34 These differences lie in large part to varying methodology in diagnosing HT, including differences in histologic definition and classification, cohort size, varying follow-up times among studies, and methods of pathologic assessment, some of which include postmortem assessment of HT or repeat biopsy.33,35,36 Despite these differences, more recent studies support the notion of a stable and consistent risk of about 2% to 3% per year through at least 10 to 15 years of diagnosis. A large population-based Canadian study of 600 patients with a median follow-up of 9 years showed the risk of transformation remained stable over time at ∼3% per year, with no evidence of plateau.26 In a large retrospective series out of the United Kingdom, 325 patients were followed for 16 years, with a similar risk of HT of about 2% to 3% per year, and a suggestion that in a subgroup of patients, risk of HT was mitigated after 16 years.34 A prospective observational study of over 600 patients with HT characterized risk in the rituximab era. With a median follow-up of 5 years, HT was reported in 11% of patients, and estimated to occur at 2% to 3% per year, consistent with earlier studies.25 Three other studies in the rituximab era by Bains et al,37 the National LymphoCare Study Group (NLCS),38 and Conconi et al39 also suggest similar frequency of HT, suggesting that incorporation of rituximab over time may not assuage the risk of HT; rather, HT is an inherent potential of at least a subset of FL, and therapy does not appear to abrogate this risk.
Determinants of risk and mitigating factors
Despite the bounty of biologic information on the pathogenesis of HT, how to best implement this knowledge into clinical practice remains unknown. Given the historically poor prognosis of patients with HT, the precise impact of various clinical factors on HT risk has been well studied in an attempt to identify the most vulnerable patients. Among these are the Follicular Lymphoma International Prognostic Index (FLIPI) as well as the International Prognostic Index (IPI), which not surprisingly have reliably predicted HT in those with higher scores among many studies. Fixed adverse clinical factors represented in the FLIPI and IPI have been independently shown to confer higher risk as well, including advanced age and LDH.25,34,38-40 Of pragmatic importance, and relevant to the practicing clinician, is whether any intervention can offset the threat of HT because no specific criteria can unequivocally predict its occurrence. Compared with expectant observation, early initiation of treatment has been suggested to decrease the risk of HT in some series,25,34,38 but not others, including a randomized study.36,41 When specifically analyzing type of treatment selected, 2 studies from the British Columbia Cancer Center suggest that the introduction of chemoimmunotherapy, in particular anthracyclines (compared with those receiving alkylator/purine combinations), as well as maintenance rituximab, led to a reduced incidence of HT.42,43 In contrast, 2 other series from the NLCS and the Spanish found no differences in development of HT among patients using doxorubicin.38,40 The completed trial conducted by Ardeshna and colleagues which randomized patients to early intensive rituximab therapy or observation has addressed the question of whether early rituximab changes HT risk. With a median follow-up of almost 4 years, no difference in the time to transformation, or incidence of HT, was detected between the 3 groups, though longer follow-up will be required.44 Based on the available literature, it is not possible to conclude definitely that early initiation of any treatment meaningfully influences the risk of HT.
Natural history of transformation, prognosis, and outcome
The prognosis of HT has varied in the literature. Prior to the use of rituximab, median overall survival (OS) of patients with HT was historically very poor, approximating 1 to 2 years across several series.2 Several more recent studies suggest this may be improving with the use of rituximab. In a large study from the University of Iowa evaluating 631 patients with FL, 60 patients developed biopsy-proven HT. In stark difference from previous eras, median OS for all patients after HT was 50 months, and at 5 years, OS was 73% in patients treated with R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine [Oncovin], prednisone) chemotherapy. This mirrored outcomes of R-CHOP–treated patients with DLCBL in a validation cohort.25 Survival following HT was similarly high in the National Comprehensive Cancer Network (NCCN) database study, which reported median OS of nearly 5 years in 118 patients with FL and biopsy confirmed HT,45 as well as for patients with early-stage FL undergoing HT, who had 3-year OS of 44%.37 Other studies also indicate that combination chemoimmunotherapy improves outcomes in patients with HT.37,46,47 Not surprisingly, patients having a complete response to treatment also fare better, as do those with early vs advanced disease at their initial diagnosis, and patients who are chemotherapy naïve at the time of transformation, compared with patients receiving prior treatments.26,27,34,45,46,48 It is unclear to what degree the timing of HT, early vs late, is clinically meaningful but in 1 study, HT < 18 months from diagnosis was associated with a worse outcome compared with later HT, with 5-year OS 22% vs 76% (P < .001).25 Precisely defining early or late timing of HT is challenging because specific time points have not been validated. Early HT may represent the manifestation of underappreciated composite histologies present at diagnosis, and many early studies describing the natural history of HT excluded HT at diagnosis.
Management challenges
Chemotherapy and transplantation
The treatment approach for a patient with HT is often individualized, as there are no randomized studies in the modern era to guide practice. Because these patients are often excluded from clinical trial participation, there is a paucity of objective data guiding optimal management of HT. The literature suggests patients treated with rituximab-containing chemotherapy can experience significantly better OS compared with other published retrospective cohorts of patients treated with chemotherapy alone, who have 5-year OS rates of 20% to 30%, and median OS ranging between 1 and 2 years.25,27 However, patients receiving dose intensification and consolidation seem to have improved outcomes. As such, the historically poor prognosis of patients with HT prompted many centers to adopt high-dose therapy (HDT) and autologous stem cell transplant (ASCT) as standard treatment in an attempt to overcome its negative prognostic impact. The role of ASCT is of continued importance in the rituximab era, as more recent studies would suggest that its incorporation into standard chemotherapy may have altered the outcome of patients with HT. To what degree chemoimmunotherapy has abrogated the need for ASCT remains a question.
Of historical relevance, patients with HT in older phase 2 and transplant registry series prior to the incorporation of immunotherapy demonstrate efficacy of ASCT,2,49 as up to 40% of patients can experience long-term benefit, with survival rates that parallel that of patients with high-grade relapsed lymphoma receiving the same treatment.50 Most transplant studies conducted in the prerituximab era are small retrospective series of between 20 and 50 patients.47,50-53 Given the heterogeneity in follow-up, inclusion of various indolent histologies, sample size, and other variables, direct comparison between these studies is problematic. Nearly all included patients were highly selected and young, with a median age in the 40s across most,51-55 though not all studies,47,56,57 and only minimal or no disease at time of ASCT. A multicenter Norwegian study of 47 patients is the only prospective series evaluating HDT and ASCT in patients with HT of FL, though conducted in the prerituximab era.57 Two-thirds of patients were chemoresponsive (in either complete remission or partial remission) and permitted to receive HDT. This study did not complete its planned accrual, though outcomes here were similar to those seen in older retrospective studies, with 5-year rates of PFS and OS of 32% and 47% (Table 1).
Studies evaluating ASCT in transformed lymphoma
| Reference . | N . | OS, % . | PFS or EFS, % . | Median follow-up, y . | Rituximab era . |
|---|---|---|---|---|---|
| 50 | 50 | 2 y, 64 | 5-y PFS. 30 Median PFS, 1.1 y | 4.9 | No |
| 5 y, 51 | |||||
| 51 | 35 | 5 y, 37 | 5-y PFS, 36 | 4.3 | No |
| 72 | 33 | 2 y, 72 | 2-y EFS, 47 | 1.7 | No |
| 5 y, 72 | 5-y EFS, 33 | ||||
| 52 | 27 | 5 y, 58 | 5-y DFS, 46 | 3.0 | No |
| 55 | 27 | Median, 8.5 y | N/A | 2.4 | No |
| 53 | 23 | 5 y, 56 | 5-y PFS, 25 | 15 | No |
| 56 | 22 | Median, 4.6 y | Median EFS, 1.4 y | 5.5 | No |
| 57 | 32 | 5 y, 32 | 5-y PFS, 47 | 6.25 | No |
| 47 | 24 | 3 y, 52 | 3-y PFS, 40 | 3.16 | No |
| 64 | 51 | 5 y, 62 | 5 y, 45 | 3 | Yes |
| 45 | 50 | 2 y, 83 | N/A | 3.4 | Yes |
| 62 | 18 | 2 y, 82 | 2-y PFS, 59 | 3.3 | Yes |
| 58 | 97 | 5 y, 65 | 5-y PFS, 55 | 7.5 | Yes |
| 60 | 50 | 3 y, 54 | 3-y PFS, 42 | 3.3 | Yes |
| 65 | 25 | 3 y, 64 | 3-y RFS, 59 | 2.1 | Yes |
| 66 | 65 | 5 y, 65 | 5-y PFS, 57 | NA | Yes |
| Reference . | N . | OS, % . | PFS or EFS, % . | Median follow-up, y . | Rituximab era . |
|---|---|---|---|---|---|
| 50 | 50 | 2 y, 64 | 5-y PFS. 30 Median PFS, 1.1 y | 4.9 | No |
| 5 y, 51 | |||||
| 51 | 35 | 5 y, 37 | 5-y PFS, 36 | 4.3 | No |
| 72 | 33 | 2 y, 72 | 2-y EFS, 47 | 1.7 | No |
| 5 y, 72 | 5-y EFS, 33 | ||||
| 52 | 27 | 5 y, 58 | 5-y DFS, 46 | 3.0 | No |
| 55 | 27 | Median, 8.5 y | N/A | 2.4 | No |
| 53 | 23 | 5 y, 56 | 5-y PFS, 25 | 15 | No |
| 56 | 22 | Median, 4.6 y | Median EFS, 1.4 y | 5.5 | No |
| 57 | 32 | 5 y, 32 | 5-y PFS, 47 | 6.25 | No |
| 47 | 24 | 3 y, 52 | 3-y PFS, 40 | 3.16 | No |
| 64 | 51 | 5 y, 62 | 5 y, 45 | 3 | Yes |
| 45 | 50 | 2 y, 83 | N/A | 3.4 | Yes |
| 62 | 18 | 2 y, 82 | 2-y PFS, 59 | 3.3 | Yes |
| 58 | 97 | 5 y, 65 | 5-y PFS, 55 | 7.5 | Yes |
| 60 | 50 | 3 y, 54 | 3-y PFS, 42 | 3.3 | Yes |
| 65 | 25 | 3 y, 64 | 3-y RFS, 59 | 2.1 | Yes |
| 66 | 65 | 5 y, 65 | 5-y PFS, 57 | NA | Yes |
EFS, event-free survival; PFS, progression-free survival.
Contemporary studies examining ASCT in the rituximab era are particularly relevant to current practice. The Canadian Bone Marrow Transplant Group (CBMTG) conducted the largest study to date, evaluating 172 patients with HT. Ninety-seven underwent ASCT. Here, ASCT improved OS and PFS of patients over rituximab-containing chemotherapy regimens, but the degree of improvement was modest (5-year OS 65% with ASCT; 61% with rituximab and chemotherapy; hazard ratio, 0.13; P < .001).58 Importantly, this study included a proportion of patients who did not receive rituximab, which may have influenced results, as likely did inclusion of patients from multiple centers. Wirk et al reported on 108 patients with biopsy-proven HT undergoing ASCT from the Center for International Blood and Marrow Transplant Research (CIBMTR). OS at 5 years was 50%, and although only 28% of patients received rituximab prior to ASCT, multivariate analysis showed no discernible impact of pretransplantation rituximab.59 Similar findings were noted after HT in 104 patients from Princess Margaret Hospital (50 proceeded to ASCT) with OS 54% at 3 years,60 and in a subgroup analysis of the prospective NCIC LY12 ASCT trial, where 4-year EFS was 45% for those undergoing ASCT, similar to that for patients with relapsed DLBCL.61 A smaller study of 18 patients with HT and prior rituximab exposure undergoing ASCT described more favorable outcomes compared with earlier series, with 2-year OS and PFS of 82% and 59%, respectively.62 Notably, patients who were rituximab naïve prior to their ASCT seemed to fare better than those with prior rituximab exposure, paralleling the observation in de novo DLBCL patients undergoing ASCT in the CORAL study.63 Reddy and colleagues studied 44 patients with HT treated with ASCT in the rituximab era in a series including patients up to the age of 70 years, as well as patients with early-stage disease and HT.64 A small fraction of patients also underwent allogeneic transplant. They found no differences between ASCT and allogeneic transplant; OS mirrored that observed in other studies, 62% at 5 years.50,53 Furthermore, patients with early HT (HT detected at diagnosis or within 1 year of diagnosis) fared significantly better, with OS of 80% at 5 years compared with those with later HT, 31%; P = .01), in contrast to findings using chemoimmunotherapy only.25
In one of the largest prospective HT cohorts in the rituximab era, patients in the NCCN database undergoing ASCT had similarly good outcomes and experienced a 2-year OS of 83%, which was superior compared with chemotherapy alone or to ASCT conducted prior to the incorporation of monoclonal antibodies and better than in other series.60,65 Notwithstanding, younger (under 60 years old) rituximab naïve patients not undergoing HDT and ASCT and treated with chemoimmunotherapy alone still fared well, with 80% OS at 5 years.45 A small subset of patients from the recently published Princess Margaret experience who did not proceed with ASCT for reasons other than disease progression also did well, experiencing 3-year OS of 65%, which is not statistically significant when compared with patients receiving ASCT (P = .330).60 This is in line with findings from a multicenter Danish registry series comparing ASCT with rituximab-containing treatment. PFS but not OS was significantly improved in patients receiving upfront ASCT (57% vs 30% at 5 years [P = .02]; 65% vs 48% [P = .11], respectively).66
Allogeneic transplantation (allo) in HT has been less well studied, with smaller numbers of patients in mostly retrospective series. For HT relapsing after ASCT, further salvage therapy with allo may improve outcomes at the expense of significant treatment-related mortality (TRM).67,68 The CBMTG series included 22 patients undergoing allo, resulting in 5 year OS of 46%, and no OS difference between allo and rituximab-containing chemotherapy (HR, 0.44; 95% CI, 0.16-1.24; P = .12).58 Data from the CIBMTR showed significant TRM in upfront allo, and poor survival that seemingly obscured any benefit gained from this modality. However reduced intensity (RIC) allo had the best results.59 RIC also seemed to benefit a select few patients relapsing after ASCT in an 18 patient study by Clavert, who reported a high 4-year OS of 68%, but with high TRM (26%) as well as graft-versus-host disease (48%).69 A British study included both patients with de novo and transformed FL and was similarly promising: 47% OS at 4 years, with no significant difference in outcomes for either group, though there was a trend toward improved survival in those with HT.70 Other reports demonstrating inferior outcomes using this modality (OS, 20%-46%) are eclipsed by formidable rates of graft-versus-host disease and TRM, but offering a small possibility of durable disease control in some.58,59,68,71,72
Radioimmunotherapy
Radioimmunotherapy (RIT) incorporating monoclonal antibodies with radioisotopes 131I tositumomab (Bexxar) or 90Y ibritumomab tiuxetan (Zevalin) has shown promise as a therapeutic agent in HT. In patients with HT, overall response rates using RIT range between 39% and 79%, with 50% of patients achieving complete remissions.49 In the largest of these, Zelenetz et al evaluated 71 patients from several 131I tositumomab studies and showed a median duration of response of 36 months in responding patients.73 An area of ongoing investigation involves the integration of RIT with HDT and transplant in transformed lymphoma, given promise that toxicities are similar to HDT alone, and has the potential to improve disease control, as well as prevent early relapse before graft-versus-lymphoma effect.74-76
Novel regimens
Because most patients with HT are excluded from clinical trials, there is limited data on novel agents in this landscape. Czuczman et al reported on a phase 2 study using lenalidomide, an immunomodulatory agent showing overall response rate of 57%, with median response duration of over 1 year in patients with HT.77 Other drugs inhibiting novel targets, such as Aurora A kinase (alisertib),78 Bruton tyrosine kinase (ibrutinib),79 the δ isoform of phosphatidylinositol 3-kinase (idelalisib),80,81 and the BCL2 protein (GDC-0199/ABT199),82 currently being studied in both indolent and aggressive lymphomas have the potential to significantly impact patients with HT. The possibility of disabling immune tolerance is also emerging as an exciting alternative to conventional therapy, particularly in light of the studies suggesting a critical role of the immune microenvironment in HT. Programmed death-1 (PD-1) is a member of the B7 receptor family that has critical functions as an immune checkpoint regulator. Pidilizumab is a monoclonal antibody to PD-1 that was recently studied following ASCT in patients with DLBCL, including a subset with HT.83
Our approach to management of transformed FL
Despite a large literature and advances in technology, no biomarker other than histologic grade has proven to be sufficiently robust to be widely adopted; many are felt to be too complex or are too subjective for general use.84 Can we then capitalize on the comprehensive genetic characterization of HT now available, for implementation into clinical practice? Notably, the 2 most prominently deregulated pathways in HT involve apoptotic resistance and epigenetic modification, 2 seemingly promising targets, for which trials are already under way (ie, GDC-0199/ABT199). Alternatively, another approach may lie in eradication of the early precursor clone by treating de novo FL, and possibly preventing HT altogether. These possibilities may inspire future clinical trial designs because optimal treatment strategies for HT remain undefined and selecting the least toxic and most efficacious therapy is the ultimate goal. Unfortunately, most clinical trials exclude patients with HT, leaving a dearth of evidence on novel treatments. We hope this paradigm can change in the future, as this clearly represents an unmet need.
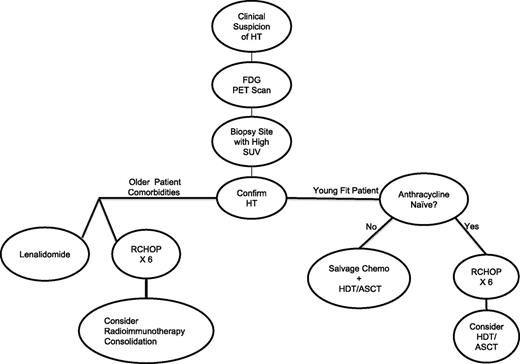
Our approach to management is detailed in Figure 2. Diagnostically, we insist that, whenever possible, excisional or needle biopsies be obtained in all patients with FL with clinical suspicion of HT. Furthermore, we consider FDG-PET imaging to help choose biopsy sites, based upon the nodal region with highest SUV. Based on our interpretation of the existing literature, if patients are anthracycline naïve, our first therapy choice is R-CHOP for 6 cycles. We then strongly consider consolidation with HDT and ASCT for younger, fit patients, particularly if they have had prior therapy for FL, given evidence suggesting this approach yields the best outcomes in the rituximab era. Selected treatment-naïve patients who obtain complete remission to R-CHOP may be approached similarly, or with rituximab maintenance in place of ASCT, though data are lacking on maintenance in this particular setting. For older patients, we have considered consolidation after R-CHOP with RIT, extrapolated from existing literature. If patients have had previous anthracycline exposure, we use aggressive platinum-containing salvage chemotherapy regimens in younger, fit patients in place of R-CHOP, followed by ASCT consolidation. For the older patient with previous anthracycline exposure, or the frail patient who cannot tolerate R-CHOP, we would consider modified or miniCHOP,85 or nonanthracycline-containing regimens such as rituximab, cyclophosphamide, etoposide, vincristine, and prednisone86 or rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisone.87 We also then consider RIT consolidation, extrapolating from single-agent activity of these drugs for transformed disease. As an alternative to chemotherapy, or if patients progress after chemotherapy, single-agent lenalidomide treatment and clinical trial participation may be considered.
Conclusions
In conclusion, HT is an important cause of morbidity and mortality in patients with FL. Given the biological heterogeneity of HT, and the relative rarity of the event, it is unlikely we will have prospective, randomized trials to guide management of this complication. However, anticipating rapid translation of genomic insights into clinical practice, the implementation of targeted DNA sequencing approaches, and a plethora of novel, targeted therapeutic agents in development, we look forward to a day soon when a molecularly defined, precision therapy approach will replace our current empiricism in the approach to HT.
Authorship
Contribution: C.C., W.R.B., and J.W.F. performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan W. Friedberg, Wilmot Cancer Institute, University of Rochester, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: jonathan_friedberg@urmc.rochester.edu.
References
Author notes
C.C. and W.R.B. contributed equally to this work.