In this issue of Blood, Kovtunovych et al report the curative effect of a nonmyeloablative bone marrow transplant for anemia and organ dysfunction in heme oxygenase 1 (HO-1)-deficient mice by restoring the ability to detoxify heme through the repopulation of the tissues with wild-type macrophages.1 These findings provide the basis for new therapies for this lethal disorder in children lacking the HO-1 gene (Hmox1).
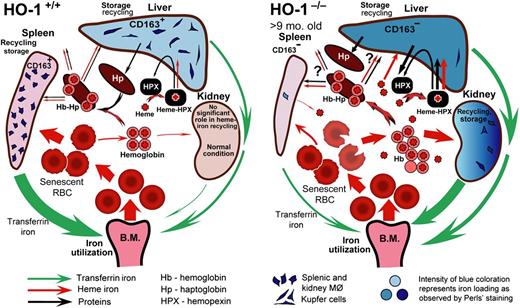
Model of heme-iron recycling and tissue iron redistribution in HO-1−/− mice. Splenic macrophages and liver Kupffer cells that phagocytose senescent red blood cells die in HO-1−/− mice. The contents released from dying macrophages include nonmetabolized heme iron. Reproduced from Figure 7 in Kovtunovych et al.6
Model of heme-iron recycling and tissue iron redistribution in HO-1−/− mice. Splenic macrophages and liver Kupffer cells that phagocytose senescent red blood cells die in HO-1−/− mice. The contents released from dying macrophages include nonmetabolized heme iron. Reproduced from Figure 7 in Kovtunovych et al.6
Yachie et al2 described a child with growth retardation, hemolytic anemia characterized by marked erythrocyte fragmentation, and intravascular hemolysis with paradoxical increase of serum haptoglobin and low bilirubin, an abnormal coagulation/fibrinolysis system, endothelial damage, asplenia, renal disease, and organ iron deposition. Sequence analysis of the patient's Hmox1 revealed complete loss of exon 2 of the maternal allele and a two-nucleotide deletion within exon 3 of the paternal allele. A lymphoid cell line derived from the patient was extremely sensitive to hemin-induced cell injury. The Kupffer cells in the patient’s liver had no heme oxygenase (HO-1). Another case of human HO-1 deficiency has been reported in a young girl with congenital asplenia who presented with severe hemolysis, inflammation, endothelial injury, and nephritis.3 Balla et al4 had shown the exquisite sensitivity of the endothelium to heme-mediated injury, which could be abrogated through the induction of HO-1 and ferritin.
Poss and Tonegawa5 showed that Hmox1−/− mice are characterized by progressive anemia, normal bilirubin, tissue iron deposition, chronic inflammation, and delayed growth similar to human patients. Furthermore, these mice are extremely sensitive to oxidative injury and are prone to death with lipopolysaccharide administration. Hmox1−/− mice were shown to lack functional splenic, hepatic, and bone marrow sinusoidal macrophages, rendering them unable to clear senescent red cells and hemoglobin from the circulation and survive erythrophagocytosis.6 Compounding the toxicity, CD163, the receptor for haptoglobin/hemoglobin, was diminished. A model was presented in this article (see figure).
Because a deficiency of macrophages able to deal with heme underlies this pathophysiology, Kovtunovych et al sought to restore HO-1-rich macrophages through a subablative bone marrow transplant (BMT). This is easier said than done, as these Hmox1−/− mice are exquisitely sensitive to oxidative stress. Wild-type bone marrow and low-dose busulfan, along with sirolimus, resulted in chimerism at 3 weeks, but most Hmox1−/− mice lost peripheral blood chimerism by the end of the experiment at 21 weeks. Despite this, the BMT normalized lactate dehydrogenase and alkaline phosphatase levels and improved hematocrits in the Hmox1−/− mice. BMT improved systemic heme-iron recycling and prevented pathological iron accumulation in kidneys, in addition to reduced systemic oxidative stress.
After BMT, Hmox1 expression was 2 to 3 times greater in the liver than in wild-type groups. Splenic Hmox1 increases, but there was negligible Hmox1mRNA in the kidney. This suggests that clearance of hemoglobin/heme in the reticuloendothelial system resulted in little free hemoglobin reaching the kidney. Remarkably, HO-1 protein was highly expressed in wild-type Kupffer-like cells in the liver of transplanted Hmox1−/− mice. The Kupffer cell localization of HO-1 is greater than hepatocyte in normal human liver. These indeed were donor Kupffer cells, which also expressed CD163.
These findings underscore the homeostatic importance of detoxifying and clearing hemoglobin and heme. The day-to-day recycling of iron and detoxification of heme is critical for hematopoiesis. The cytoprotective aspects of HO-1 in hemolysis can be seen in sickle cell disease, where gene therapy augmenting HO-1 can modulate microvascular occlusion in murine models of sickle cell disease and where HO-1 polymorphisms are associated with acute chest syndrome.7,8
Could bone marrow transplants be used to treat children with HO-1 deficiency? Certainly these data suggest this possibility. In addition, gene therapy, induced pluripotent stem cells, and possibly gene editing of CD34 hematopoietic stem cells, followed by autologous transplants, may be on the horizon. HO-1 deficiency also may be more common than previously documented. The triad of intravascular hemolysis, high haptoglobin, and low bilirubin certainly should alert clinicians regarding HO-1 deficiency.
How the Hmox1−/− mouse livers become so efficiently repopulated with homing wild-type Kupffer cells is still a mystery. This niche is “open” in the Hmox1−/− mice, as liver macrophages are absent and the wild-type macrophages may just move in. Could the engraftment of wild-type macrophages nurse the bone marrow in these Hmox1−/− mice to correct the anemia? When “Mac” the macrophage is back in town, heme is detoxified and iron is recycled.
Conflict-of-interest disclosure: The author declares no competing financial interests