In this issue of Blood, Taur et al demonstrate that a lack of intestinal microbial diversity independently predicts nonrelapse mortality (NRM) in allogeneic hematopoietic cell transplant recipients.1 At the time of engraftment, patients with low microbial diversity were at fivefold higher risk for NRM than patients with high microbial diversity, primarily because of graft-versus-host disease (GVHD).
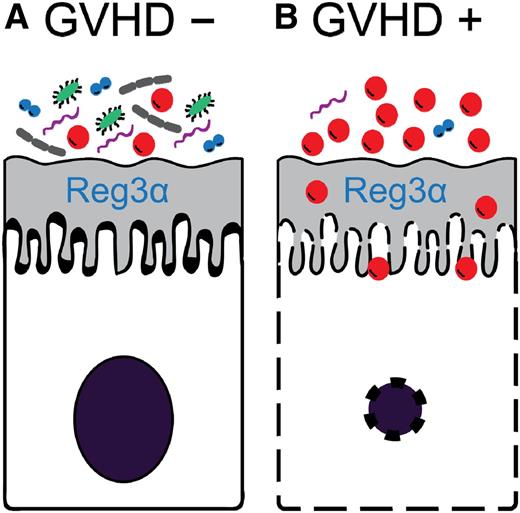
Hypothetical model of the relationship between intestinal bacterial diversity and GVHD. (A) When bacterial diversity is high, antimicrobial peptides such as Reg3α can protect intestinal epithelial cells from damage and GVHD is averted. (B) When bacterial diversity is low, Reg3α is insufficient to counter the overwhelming number of bacteria resulting in intestinal epithelial cell apoptosis and GVHD.
Hypothetical model of the relationship between intestinal bacterial diversity and GVHD. (A) When bacterial diversity is high, antimicrobial peptides such as Reg3α can protect intestinal epithelial cells from damage and GVHD is averted. (B) When bacterial diversity is low, Reg3α is insufficient to counter the overwhelming number of bacteria resulting in intestinal epithelial cell apoptosis and GVHD.
GVHD remains the major cause of morbidity and mortality following allogeneic hematopoietic cell transplant and limits its use as a curative therapy for malignant and nonmalignant hematologic diseases. The gastrointestinal (GI) tract is affected in nearly all cases of severe GVHD,2 and thus better prevention and control of GI GVHD is essential to reducing NRM. Recent studies show that the GI microbiome plays a role in orchestrating the immune responses that culminate in both experimental and human GVHD.3,4 The detection of specific microbes by Toll-like receptors on intestinal epithelial cells triggers an inflammatory response that leads to both the recruitment of T cells to the GI tract and activation of the antigen-presenting cells that drive the adaptive immune response.5 Damage to the intestinal epithelial barrier, either by pathogens or the pretransplant conditioning regimen, results in translocation of bacteria and their byproducts that can further damage to the intestinal epithelium.6 The intestinal mucosal barrier protects itself from such damage by the secretion of antimicrobial peptides, which are primarily produced by Paneth cells and which regulate the composition of the microbiome and maintain intestinal health. When these peptides are in short supply, intestinal homeostasis is disrupted and pathogenic bacterial species can predominate. The role of these antimicrobial peptides in clinical GVHD was confirmed when Reg3α was validated as a GI GVHD biomarker.7 The important role played by regulators of bacterial composition in GVHD is further supported by the observation that Paneth cell loss correlates with higher NRM in both experimental and clinical allogeneic hematopoietic cell transplantation.3,8
If regulation of commensal bacteria is important to GI GVHD control, then one would predict that the loss of regulation and the subsequent bacterial species overgrowth and loss of diversity would increase the risk of lethal GVHD. It is in this context that the present study is particularly significant because it correlates loss of diversity with lack of GVHD control and death. It is likely that overgrowth of specific bacterial pathogens overwhelms the defenses of the intestinal mucosa barrier, resulting in apoptosis of intestinal epithelial cells and permitting gaps for the translocation of the bacterial products that activate the adaptive immune system and promote GVHD (see figure). It is also possible that the bacterial metabolites play an important role in modulating GI mucosal immunity. For example, short chain fatty acids such as butyrate, which are generated by anaerobic bacteria in the intestine, are known to increase the number of regulatory T cells.9 Regulatory T cells can reduce GVHD.10 Thus, the overall composition of the intestinal microbiome may modulate GI epithelial health through a variety of mechanisms.
This work has important clinical implications. It suggests that maintenance or restoration of intestinal bacterial homeostasis may prevent or control GI GVHD. The correlation of low bacterial diversity at a single assessment, the period surrounding engraftment, with transplant-related deaths that occurred months later is notable and raises the question as to whether an intervention early after transplant would be effective. One might explore the more selective use of antibiotics, administration of probiotics, or even fecal transplants as strategies to achieve higher rates of intestinal bacterial diversity. In that regard, the finding that bacterial diversity was lowest during active GI GVHD but reverted to normal when symptoms improved supports examining such strategies.4
We do not yet know why bacterial diversity is so variable in allogeneic hematopoietic cell transplant recipients. Taur et al report that patients who received intensive conditioning regimens were more likely to have lower bacterial diversity, but their study population was not sufficiently large to identify other risk factors. The variation may therefore be due, in part, to the intensity of therapy prior to transplantation; intensive regimens are more likely to induce febrile neutropenia and subsequent use of broad spectrum antibiotics, which can profoundly reduce intestinal diversity. A better understanding of the risk factors for low bacterial diversity is still needed to design the best clinical trials.
Conflict-of-interest statement: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal