Key Points
Weekly oral proteasome inhibitor ixazomib appears generally well tolerated with manageable toxicity, limited grade 1/2 neuropathy.
Data show that more than 25% of 30 evaluable relapsed/refractory myeloma patients who received the MTD had clinically meaningful responses.
Abstract
Proteasome inhibition is an effective treatment strategy for multiple myeloma. With improving survival, attention is increasingly focusing on ease of administration and toxicity profile. Ixazomib is an investigational, orally bioavailable 20S proteasome inhibitor. Sixty patients with relapsed and/or refractory multiple myeloma were enrolled on this phase 1 trial to evaluate safety and tolerability and determine the maximum tolerated dose (MTD) of single-agent, oral ixazomib given weekly for 3 of 4 weeks. Upon MTD determination, patients were enrolled to 4 different cohorts based on relapsed/refractory status and prior bortezomib and carfilzomib exposure. The MTD was determined to be 2.97 mg/m2. Dose-limiting toxicities were grade 3 nausea, vomiting, and diarrhea in 2 patients, and grade 3 skin rash in 1 patient. Common drug-related adverse events were thrombocytopenia (43%), diarrhea (38%), nausea (38%), fatigue (37%), and vomiting (35%). The observed rate of peripheral neuropathy was 20%, with only 1 grade 3 event reported. Nine (18%) patients achieved a partial response or better, including 8 of 30 (27%) evaluable patients treated at the MTD. Pharmacokinetic studies suggested a long terminal half-life of 3.6 to 11.3 days, supporting once-weekly dosing. This trial was registered at www.clinicaltrials.gov as #NCT00963820.
Introduction
Treatment paradigms in multiple myeloma (MM) are rapidly evolving as a result of better understanding of the underlying biology of the disease and the introduction of new, effective drugs such as proteasome inhibitors (PIs) and immunomodulatory drugs.1-5 A treatment strategy of using these drugs alone and in combinations has led to deeper responses and more durable disease control,6,7 resulting in significantly improved survival.6-9
Bortezomib was the first PI to be tested in the clinic; clinical trials have demonstrated its effectiveness in treating both newly diagnosed10-15 and relapsed and/or refractory MM.16-19 Bortezomib has been combined with other MM drugs leading to several highly effective combination regimens.11-15,20 In particular, bortezomib plays a critical role in the management of patients with genetically high-risk MM14,21-23 and patients with renal insufficiency.24,25 The introduction of subcutaneous administration has improved convenience compared with the intravenous approach and substantially reduced the risk of peripheral neuropathy (PN),26 a major limiting factor for long-term administration.27 Other studies have shown that once-weekly administration of bortezomib can significantly reduce the risk of PN while preserving efficacy.28,29 Given this context, the development of an orally bioavailable PI with an improved toxicity profile would represent a major advance for MM therapy.
Ixazomib is an investigational small molecule 20S PI30 and is the first orally bioavailable PI to be tested in the clinic. Ixazomib (MLN2238) refers to the biologically active boronic acid form of ixazomib citrate (MLN9708), the drug substance, which is administered as a stable citrate ester.30 This undergoes rapid hydrolysis to ixazomib under physiological conditions.30 Ixazomib preferentially binds the β5 site of the 20S proteasome at lower doses, with inhibition of the β1 and β2 sites at higher concentrations. Compared with bortezomib, nonclinical studies have shown that ixazomib has a faster dissociation rate from the proteasome.30 Ixazomib has demonstrated antitumor activity in a range of tumor xenograft models, including MM models.30-33 Preclinical studies have shown activity in myeloma cells resistant to bortezomib.31 Additionally, ixazomib has shown synergistic antimyeloma activity combined with dexamethasone and lenalidomide in preclinical studies.31 These encouraging preclinical data formed the basis for clinical evaluation of ixazomib.
Patients and methods
Study design
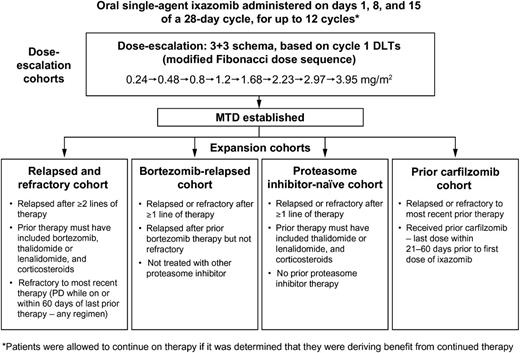
This open-label, dose-escalation phase 1 study evaluated the safety and tolerability of weekly oral ixazomib in patients with relapsed and/or refractory MM. Patients were enrolled at 6 sites in the United States between November 2009 and September 2012. The study consisted of a dose-escalation phase to determine the maximum tolerated dose (MTD), followed by multiple expansion cohorts treated at the MTD, with patients selected for individual cohorts based on prior therapy (Figure 1). The study was performed in accordance with the provisions of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice, and with approval of the institutional review boards at individual enrolling institutions.
Summary of study design, including dose-escalation phase and eligibility criteria for the 4 expansion cohorts. IMiD, immunomodulatory drug; PD, progressive disease.
Summary of study design, including dose-escalation phase and eligibility criteria for the 4 expansion cohorts. IMiD, immunomodulatory drug; PD, progressive disease.
Study objectives
The primary objective was to determine the safety profile, tolerability, and MTD of oral ixazomib on a weekly dosing schedule. The secondary objectives included characterization of ixazomib pharmacokinetics in plasma, and assessment of overall response rate (ORR) and rate of minimal response (MR) or better.
Patient selection
Patients were eligible for the dose-escalation phase if they had relapsed or refractory MM after at least 2 prior lines of therapy, which must have included bortezomib, thalidomide or lenalidomide, and corticosteroids in any combination. Criteria for enrollment to the MTD expansion cohorts differed only in terms of prior therapies and relapsed/refractory status (Figure 1). Patients could be refractory to any regimen as most recent prior therapy, except where specified.
Patients required measurable disease (serum M-protein ≥1 g/dL [≥0.5 g/dL acceptable in dose-escalation phase] or urine M-protein ≥200 mg/24 hours), Eastern Cooperative Oncology Group performance status of 0-2, adequate hematologic (absolute neutrophil count ≥1,000/mm3, platelets ≥75 000/mm3), hepatic (total bilirubin ≤1.5 × upper limit of normal, alanine/aspartate aminotransferase ≤2.5 × upper limit of normal), and renal (creatinine clearance ≥20 mL per minute) function. Patients with grade ≥2 PN (per other studies of PIs13,17,34,35 ) or grade >1 diarrhea, or who had major surgery, serious infection, radiotherapy, or systemic treatment with strong CYP1A2 inhibitors or strong inhibitors/inducers of CYP3A within 14 days, or any investigational products within 21 days of the first dose of ixazomib, were excluded. No prior exposure to investigational PIs was allowed, except for carfilzomib. Concurrent corticosteroid therapy for coexisting conditions in excess of 10 mg per day prednisone or equivalent was prohibited. Other comorbidities or severe preexisting illness that in the treating physician’s opinion could interfere with oral absorption and/or tolerance of ixazomib excluded patients from participation.
Drug administration
Ixazomib was administered orally on days 1, 8, and 15 of a 28-day cycle for up to 12 cycles or until disease progression or unacceptable toxicity, but could be continued in the setting of clinical benefit. Dose escalation proceeded via a standard 3+3 design based upon occurrence of dose-limiting toxicities (DLTs) in cycle 1 (Figure 1). DLTs were defined as 1 or more of the following toxicities considered related to ixazomib: (1) grade 4 neutropenia lasting >7 days or grade 3 neutropenia with fever (≥38.5°C) and/or infection; (2) grade 4 thrombocytopenia lasting >7 days, grade 3 thrombocytopenia with clinically significant bleeding, or platelets <10 000/mm3 at any time; (3) any grade ≥3 nonhematologic toxicity except grade 3 arthralgia/myalgia and brief (<1 week) grade 3 fatigue; (4) delay of ≥2 weeks in starting cycle 2 because of lack of recovery from ixazomib-related toxicities in cycle 1; (5) grade 2 PN with pain; or (6) other ixazomib-related grade ≥2 nonhematologic toxicities requiring drug discontinuation. The MTD was the highest dose level with no more than 1 patient experiencing DLTs during cycle 1. Standard supportive care measures were allowed for management of nausea and diarrhea; prophylactic antiemetics were not allowed during cycle 1 or routinely recommended. Topical steroids and other symptomatic measures were permitted for management of skin rash.
Assessments
Adverse events (AEs) were graded using the National Cancer Institute’s Common Terminology Criteria for AEs, version 4.0. Myeloma disease response was done in accordance with the International Myeloma Working Group uniform criteria,36 incorporating the additional categories of MR37 and near-complete response.16 The individual investigators performed response assessments.
Detailed pharmacokinetic analyses were incorporated. Blood samples (3 mL) were taken before dosing (within 1 hour) on days 1, 8, and 15 of cycle 1; day 1 of cycle 2; and at 15 and 30 minutes and 1, 1.5, 2, 4, 8, 24, 48, and 96 hours after ixazomib administration on days 1 and 15 of cycle 1. Ixazomib plasma concentrations were measured using a validated liquid chromatography–mass spectrometry assay.
Statistical analyses
For toxicity assessment, the safety population was defined as patients receiving at least 1 dose of ixazomib. The DLT-evaluable population included patients receiving all ixazomib doses in cycle 1 and either completing cycle 1 or experiencing a DLT during cycle 1. Patients receiving all protocol-specified ixazomib doses in cycle 1, with no disallowed concomitant medications, and having sufficient concentration–time data to permit reliable estimation of pharmacokinetic parameters were included in the pharmacokinetic analyses. All patients who received at least 1 dose of ixazomib and had measurable disease at baseline and at least 1 postbaseline disease assessment were considered evaluable for disease response. Data were summarized using descriptive statistics; no formal comparisons were performed between expansion cohorts. Patients from the dose-escalation MTD cohort were included in the expansion cohorts if they met the eligibility criteria for the respective cohort.
Results
Patient enrollment
Sixty patients were enrolled. There were 32 patients enrolled across 8 dose levels in the dose-escalation phase, including 3 patients each at 0.24, 0.48, 0.8, and 1.2 mg/m2, 4 at 1.68 mg/m2, 3 at 2.23 mg/m2, 8 at 2.97 mg/m2, and 5 at 3.95 mg/m2. Subsequently, a further 28 patients were enrolled to the expansion cohorts, and 3 patients treated at 2.97 mg/m2 (MTD) in the dose-escalation cohort were included in the relapsed and refractory (n = 2) and bortezomib-relapsed (n = 1) expansion cohorts. In total, 11, 10, 6, and 4 patients were included in the relapsed and refractory, bortezomib-relapsed, PI-naive, and carfilzomib cohorts, respectively. Patients had received a median of 6 prior regimens over a median of 4.9 years since MM diagnosis. Table 1 summarizes patients’ characteristics.
Demographics and baseline characteristics of patients enrolled to the dose-escalation cohorts and the expansion cohorts
| Characteristic . | Dose-escalation cohorts (n = 32) . | Expansion cohorts (n = 31*) . | Total (N = 60) . |
|---|---|---|---|
| Median age, y (range) | 64.0 (40-76) | 65.0 (40-79) | 64.0 (40-79) |
| Male, n (%) | 17 (53) | 18 (58) | 33 (55) |
| Race, n (%) | |||
| White | 25 (78) | 29 (94) | 51 (85) |
| African American | 7 (22) | 0 | 7 (12) |
| Other | 0 | 2 (6) | 2 (3) |
| MM subtype, n (%) | |||
| IgG | 18 (56) | 24 (77) | 41 (68) |
| IgA | 9 (28) | 3 (10) | 10 (17) |
| Light chain | 5 (16) | 4 (13) | 9 (15) |
| ISS disease stage, n (%) | |||
| I | 13 (41) | 5 (16) | 18 (30) |
| II | 13 (41) | 18 (58) | 29 (48) |
| III | 5 (16) | 7 (23) | 12 (20) |
| Unknown | 1 (3) | 1 (3) | 1 (3) |
| Median β2-microglobulin, mg/L (range) | 3.80 (1.6-11.3) | 4.15 (1.8-8.0) | 3.80 (1.6-11.3) |
| Median creatinine clearance, mL/min (range) | 74.46 (30.8-179.5) | 78.75 (26.0-214.5) | 76.55 (26.0-214.5) |
| Creatinine clearance <50 mL/min, n (%)† | 3 (9) | 7 (23) | 10 (17) |
| Patients with cytogenetic assessment, n | 30 | 28 | 55 |
| Cytogenetics by FISH, n/N (%) | 24/30 (80) | 23/28 (82) | 44/55 (80) |
| Unfavorable cytogenetic abnormalities, n (%) | |||
| t(4;14) | 2 (8) | 3 (13) | 4 (9) |
| t(14;16) | 0 | 1 (4) | 1 (2) |
| −17p | 2 (8) | 1 (4) | 3 (7) |
| Median time since MM diagnosis, years (range) | 4.9 (1.5-18.8) | 4.9 (1.7-12.3) | 4.9 (1.5-18.8) |
| Median number of prior lines of therapy, n (range) | 4 (1‡-13) | 3 (1-12) | 4 (1-13) |
| Prior therapy with: n (%) | |||
| Bortezomib | 31 (97)‡ | 23 (74) | 51 (85) |
| Lenalidomide | 30 (94) | 31 (100) | 58 (97) |
| Thalidomide | 19 (59) | 15 (48) | 32 (53) |
| Carfilzomib | 4 (13) | 5 (16) | 9 (15) |
| Prior SCT, n (%) | 23 (72) | 25 (81) | 46 (77) |
| Refractory to last prior therapy, n (%) | 17 (57)§ | 27 (87) | 42 (72)§ |
| Bortezomib-refractory, n (%) | 7 (22) | 5 (16) | 11 (18) |
| Lenalidomide/thalidomide-refractory, n (%) | 11 (34) | 13 (42) | 23 (38) |
| Characteristic . | Dose-escalation cohorts (n = 32) . | Expansion cohorts (n = 31*) . | Total (N = 60) . |
|---|---|---|---|
| Median age, y (range) | 64.0 (40-76) | 65.0 (40-79) | 64.0 (40-79) |
| Male, n (%) | 17 (53) | 18 (58) | 33 (55) |
| Race, n (%) | |||
| White | 25 (78) | 29 (94) | 51 (85) |
| African American | 7 (22) | 0 | 7 (12) |
| Other | 0 | 2 (6) | 2 (3) |
| MM subtype, n (%) | |||
| IgG | 18 (56) | 24 (77) | 41 (68) |
| IgA | 9 (28) | 3 (10) | 10 (17) |
| Light chain | 5 (16) | 4 (13) | 9 (15) |
| ISS disease stage, n (%) | |||
| I | 13 (41) | 5 (16) | 18 (30) |
| II | 13 (41) | 18 (58) | 29 (48) |
| III | 5 (16) | 7 (23) | 12 (20) |
| Unknown | 1 (3) | 1 (3) | 1 (3) |
| Median β2-microglobulin, mg/L (range) | 3.80 (1.6-11.3) | 4.15 (1.8-8.0) | 3.80 (1.6-11.3) |
| Median creatinine clearance, mL/min (range) | 74.46 (30.8-179.5) | 78.75 (26.0-214.5) | 76.55 (26.0-214.5) |
| Creatinine clearance <50 mL/min, n (%)† | 3 (9) | 7 (23) | 10 (17) |
| Patients with cytogenetic assessment, n | 30 | 28 | 55 |
| Cytogenetics by FISH, n/N (%) | 24/30 (80) | 23/28 (82) | 44/55 (80) |
| Unfavorable cytogenetic abnormalities, n (%) | |||
| t(4;14) | 2 (8) | 3 (13) | 4 (9) |
| t(14;16) | 0 | 1 (4) | 1 (2) |
| −17p | 2 (8) | 1 (4) | 3 (7) |
| Median time since MM diagnosis, years (range) | 4.9 (1.5-18.8) | 4.9 (1.7-12.3) | 4.9 (1.5-18.8) |
| Median number of prior lines of therapy, n (range) | 4 (1‡-13) | 3 (1-12) | 4 (1-13) |
| Prior therapy with: n (%) | |||
| Bortezomib | 31 (97)‡ | 23 (74) | 51 (85) |
| Lenalidomide | 30 (94) | 31 (100) | 58 (97) |
| Thalidomide | 19 (59) | 15 (48) | 32 (53) |
| Carfilzomib | 4 (13) | 5 (16) | 9 (15) |
| Prior SCT, n (%) | 23 (72) | 25 (81) | 46 (77) |
| Refractory to last prior therapy, n (%) | 17 (57)§ | 27 (87) | 42 (72)§ |
| Bortezomib-refractory, n (%) | 7 (22) | 5 (16) | 11 (18) |
| Lenalidomide/thalidomide-refractory, n (%) | 11 (34) | 13 (42) | 23 (38) |
FISH, fluorescence in situ hybridization; Ig, immunoglobulin; SCT, stem cell transplantation.
Includes 3 patients from the MTD dose-escalation cohort.
Only 1 patient in the expansion cohorts had creatinine clearance of ≤30 mL/minute.
Protocol violation.
Data missing for 2 patients.
All patients received at least 1 dose of ixazomib and were included in the safety population. The DLT-evaluable population consisted of 29 patients, with 3 excluded for not receiving all doses during cycle 1 in the absence of DLT. For pharmacologic studies, 44 patients were included in the pharmacokinetics population. Fifty patients were included in the response-evaluable population (10 excluded because of absence of measurable disease at baseline [n = 6] or postbaseline assessment [n = 4]). At data cutoff for this article (March 1, 2013), 3 patients in the expansion cohorts were continuing to receive study treatment in cycles 6, 11, and 12; 57 patients have discontinued because of progressive disease (n = 42, 70%), AEs (n = 7, 12%), patient withdrawal (n = 6, 10%), or unsatisfactory therapeutic response (n = 2, 3%).
DLTs and MTD
There were 3 DLTs observed across the dose-escalation phase: 1 of 6 DLT-evaluable patients treated at 2.97 mg/m2 and 2 of 4 DLT-evaluable patients treated at 3.95 mg/m2. One patient treated at 2.97 mg/m2 experienced grade 3 nausea, vomiting, and diarrhea on day 12 of cycle 1 leading to treatment interruption and hospitalization. After resolution of these AEs, the patient continued at a lower ixazomib dose. The first DLT at 3.95 mg/m2 consisted of grade 3 nausea, vomiting, and diarrhea on days 9-10 of cycle 1, which led to dose interruption and hospitalization. After resolution of the AEs, the patient continued at a reduced dose for subsequent cycles. The second DLT at 3.95 mg/m2 was the development of grade 3 erythema multiforme rash on day 11 of cycle 1, resulting in a dosing delay and dose reduction of ixazomib. The rash subsequently resolved and the patient continued therapy on a reduced dose. Based on the observed DLTs, the MTD of weekly oral ixazomib was determined to be 2.97 mg/m2; this dose was used in the expansion cohorts.
Treatment exposure
At data cutoff, with 3 patients remaining on study, the median number of cycles administered was 2 (range 1 to 12, mean 3.7), including a median of 2 (range 1-11) in the dose-escalation cohorts and 3 (range 1-12) in the expansion cohorts. Among all 60 patients, 19 (32%) were treated for ≥4 cycles, 11 (18%) for ≥8 cycles, and 5 (8%) for ≥11 cycles, including 1 (2%) patient who received 12 cycles. Patients received a median cumulative dose of 29.7 mg of ixazomib (range 1-179 mg) at a median dose intensity of 6.5 mg/m2 per cycle (range 0-11, mean 5.9 mg/m2 per cycle). Among patients treated at the MTD, median dose intensity was 7.9 mg/m2 per cycle (range 4- 9), representing 89% of that planned (8.91 mg/m2). Estimated dosing compliance was 94% (mean of 13.0 doses received during a mean treatment duration of 4.6 cycles, equating to 13.8 doses).
Toxicity profile
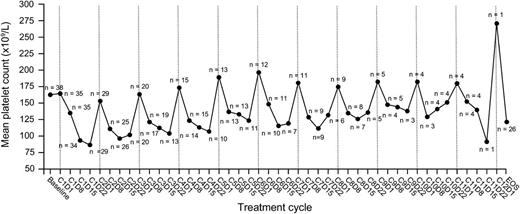
Among all 60 patients, 59 (98%) experienced at least 1 treatment-emergent AE, and 39 (65%) had at least 1 grade ≥3 treatment-emergent AE. Drug-related AEs and drug-related grade ≥3 AEs were seen in 51 (85%) and 32 (53%) patients, respectively. Common drug-related AEs of any grade and drug-related grade ≥3 AEs are summarized in Table 2. Supplemental Table 1 on the Blood Web site summarizes all-cause AEs and drug-related AEs by grade in all 60 patients. Drug-related AEs appeared generally similar in patients with renal impairment (creatinine clearance <50 mL per minute, n = 10; supplemental Table 2). Drug-related grade 4 AEs were observed in 11 (18%) patients, including 3 of 32 (9%) and 9 of 31 (29%) patients in the dose-escalation and expansion cohorts, respectively. These included thrombocytopenia in 9 (15%) patients and congestive cardiac failure, hyperuricemia, neutropenia, and lymphopenia in 1 (2%) patient each. Thrombocytopenia appeared transient and cyclical (Figure 2). Platelet transfusions were required in 5 patients.
Most commonly reported all-grade (≥10% of patients overall) and grade ≥3 (≥2 patients) drug-related AEs
| AE, n (%) . | Dose-escalation cohorts (n = 32) . | Expansion cohorts (n = 31*) . | Total (N = 60) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3† . | |
| Any | 26 (81) | 13 (41) | 28 (90) | 22 (71) | 51 (85) | 32 (53) |
| Thrombocytopenia | 14 (44) | 9 (28) | 14 (45) | 12 (39) | 26 (43) | 20 (33) |
| Diarrhea | 11 (34) | 4 (13) | 14 (45) | 7 (23) | 23 (38) | 10 (17) |
| Nausea | 11 (34) | 3 (9) | 13 (42) | 1 (3) | 23 (38) | 4 (7) |
| Fatigue | 12 (38) | 2 (6) | 13 (42) | 4 (13) | 22 (37) | 5 (8) |
| Vomiting | 9 (28) | 2 (6) | 13 (42) | 1 (3) | 21 (35) | 3 (5) |
| Decreased appetite | 5 (16) | 0 | 11 (35) | 4 (13) | 15 (25) | 4 (7) |
| Neutropenia | 4 (13) | 4 (13) | 10 (32) | 8 (26) | 13 (22) | 11 (18) |
| Skin/SC tissue disorders‡ | 3 (9) | 1 (3)§ | 11 (35) | 1 (3)|| | 13 (22) | 2 (3) |
| PN NEC¶ | 3 (9) | 0 | 10 (32) | 1 (3) | 12 (20) | 1 (2) |
| Anemia | 3 (9) | 2 (6) | 7 (23) | 2 (6) | 9 (15) | 4 (7) |
| Dehydration | 3 (9) | 1 (3) | 5 (16) | 1 (3) | 7 (12) | 2 (3) |
| Lymphopenia | 3 (9) | 3 (9) | 5 (16) | 3 (10) | 7 (12) | 5 (8) |
| Leukopenia | 2 (6) | 1 (3) | 5 (16) | 3 (10) | 6 (10) | 3 (5) |
| Pyrexia | 1 (3) | 0 | 5 (16) | 0 | 6 (10) | 0 |
| AE, n (%) . | Dose-escalation cohorts (n = 32) . | Expansion cohorts (n = 31*) . | Total (N = 60) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade ≥3 . | All grades . | Grade ≥3 . | All grades . | Grade ≥3† . | |
| Any | 26 (81) | 13 (41) | 28 (90) | 22 (71) | 51 (85) | 32 (53) |
| Thrombocytopenia | 14 (44) | 9 (28) | 14 (45) | 12 (39) | 26 (43) | 20 (33) |
| Diarrhea | 11 (34) | 4 (13) | 14 (45) | 7 (23) | 23 (38) | 10 (17) |
| Nausea | 11 (34) | 3 (9) | 13 (42) | 1 (3) | 23 (38) | 4 (7) |
| Fatigue | 12 (38) | 2 (6) | 13 (42) | 4 (13) | 22 (37) | 5 (8) |
| Vomiting | 9 (28) | 2 (6) | 13 (42) | 1 (3) | 21 (35) | 3 (5) |
| Decreased appetite | 5 (16) | 0 | 11 (35) | 4 (13) | 15 (25) | 4 (7) |
| Neutropenia | 4 (13) | 4 (13) | 10 (32) | 8 (26) | 13 (22) | 11 (18) |
| Skin/SC tissue disorders‡ | 3 (9) | 1 (3)§ | 11 (35) | 1 (3)|| | 13 (22) | 2 (3) |
| PN NEC¶ | 3 (9) | 0 | 10 (32) | 1 (3) | 12 (20) | 1 (2) |
| Anemia | 3 (9) | 2 (6) | 7 (23) | 2 (6) | 9 (15) | 4 (7) |
| Dehydration | 3 (9) | 1 (3) | 5 (16) | 1 (3) | 7 (12) | 2 (3) |
| Lymphopenia | 3 (9) | 3 (9) | 5 (16) | 3 (10) | 7 (12) | 5 (8) |
| Leukopenia | 2 (6) | 1 (3) | 5 (16) | 3 (10) | 6 (10) | 3 (5) |
| Pyrexia | 1 (3) | 0 | 5 (16) | 0 | 6 (10) | 0 |
PN NEC, peripheral neuropathy not elsewhere classified; SC, subcutaneous.
Includes 3 patients from the MTD dose-escalation cohort.
In addition, the following drug-related grade ≥3 AEs were reported in 1 patient each: hypocalcemia, hyperuricemia, hyponatremia, decreased platelet count, increased blood creatinine, decreased white blood cell count, dizziness, erythema multiforme, rash papular, cardiac failure congestive, pneumonia, renal failure acute, and orthostatic hypotension.
Skin/SC tissue disorders cover all AEs within this MedDRA system organ class; overall rate includes rash macular (n = 3, 5%), hyperhidrosis, exfoliative rash (each n = 2, 3%), acute febrile neutrophilic dermatosis, alopecia, erythema multiforme, night sweats, petechiae, rash, rash erythematous, rash papular, skin exfoliation, and an event coded as Stevens–Johnson syndrome with a clinical diagnosis of erythema multiforme (each n = 1, 2%).
DLT of grade 3 erythema multiforme.
Grade 3 rash papular.
PN NEC (high-level MedDRA term, including the preferred terms of “neuropathy peripheral,” “peripheral sensory neuropathy,” and “peripheral motor neuropathy”).
Mean platelet count in all patients treated at the ixazomib MTD of 2.97 mg/m2. Thrombocytopenia appeared transient and cyclical, with recovery of platelet count toward baseline in the rest period at the end of each treatment cycle.
Mean platelet count in all patients treated at the ixazomib MTD of 2.97 mg/m2. Thrombocytopenia appeared transient and cyclical, with recovery of platelet count toward baseline in the rest period at the end of each treatment cycle.
PN of any type considered drug-related was seen in 12 (20%) patients, including 3 (9%) patients in the dose-escalation cohort and 10 (32%) patients in the expansion cohorts. Among these, 4 had grade 1, 7 had grade 2, and 1 had grade 3 PN. The grade 3 event was seen in a patient in the prior carfilzomib cohort who had also previously received thalidomide but did not have PN at baseline. The patient noticed PN during cycle 3. The event resulted in an ixazomib dosing hold that was ongoing at the time of last follow-up; the patient subsequently discontinued ixazomib (patient withdrawal). Among the other 11 patients, 5 had grade 1 PN at baseline; 4 patients (treated at 0.24 mg/m2, 3.95 mg/m2, and in the relapsed and refractory, and prior carfilzomib cohorts) had worsening to grade 2 and 1 patient in the PI-naive cohort reported worsening but was still grade 1. Of the remaining 6 patients who did not have PN at baseline, 3 (treated in the 2.97 mg/m2 MTD dose-escalation cohort and in the bortezomib-relapsed and prior carfilzomib cohorts) reported grade 1 PN and 3 (2 in the relapsed and refractory cohort, 1 in the bortezomib-relapsed cohort) reported grade 2 PN. The onset of PN varied considerably and was seen during cycles 1 through 5; to date, PN AEs have resolved in 3 patients.
A total of 19 drug-related serious AEs were reported in 11 (18%) patients, including 3 (9%) and 9 (29%) patients in the dose-escalation and expansion cohorts, respectively. These included diarrhea in 5 (8%) patients, vomiting in 3 (5%), nausea in 2 (3%), dehydration in 2 (3%), pneumonia in 2 (3%), and dyspnea, renal failure acute (prerenal), orthostatic hypotension, an event coded as Stevens–Johnson syndrome with a clinical diagnosis of bullous erythema multiforme, and congestive heart failure in 1 (2%) patient each.
AEs led to ixazomib dose reductions in 19 (32%) patients, including 6 of 32 (19%) patients in the dose-escalation cohort, and 14 of 31 (45%) patients in the expansion cohorts at the MTD; 5 (8%) patients required ≥2 dose reductions. The most common AEs resulting in dose reductions included thrombocytopenia, diarrhea (each 12%), nausea (5%), decreased appetite, dehydration, neutropenia, and vomiting (each 3%). Seven (12%) patients discontinued ixazomib because of AEs including renal failure/blood creatinine increase (n = 2), thrombocytopenia, diarrhea, nausea and vomiting, hypercalcemia, and dyspnea (each n = 1). One patient, treated at 2.97 mg/m2, died while on the study from complications related to disease progression.
Disease response
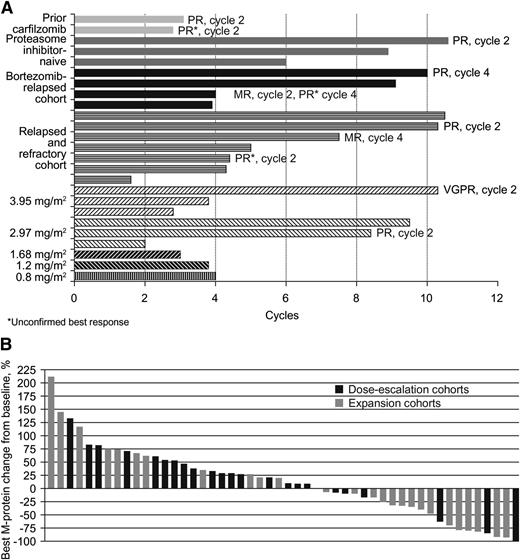
Among 50 evaluable patients, 10 (20%) had best responses of MR or better, including 1 very good partial response, 8 partial responses (PRs), and 1 MR (Table 3). In another 15 (30%) patients, the best response seen was stable disease (SD). Among 30 response-evaluable patients treated at the MTD, 8 achieved a PR, for an ORR of 27%. The duration of SD or better among patients achieving SD or better is shown in Figure 3A; individual patient’s best M-protein responses are shown in Figure 3B. The median duration of SD or better among the 25 response-evaluable patients achieving SD or better was 4.0 months (range 1.4+ to 9.8+) and for the 10 patients achieving an MR or better was 7.3 months (range 2.6+ to 9.8+).
Confirmed or unconfirmed best responses to ixazomib by investigator assessment
| Response . | Dose-escalation cohorts (n = 23) . | Expansion cohorts (n = 30*) . | Total (N = 50) . |
|---|---|---|---|
| Best response, n (%) | |||
| CR | 0 | 0 | 0 |
| PR | 2 (9) | 8 (27)† | 9 (18)† |
| VGPR | 1 (4) | 0 | 1 (2) |
| MR | 0 | 1 (3) | 1 (2) |
| SD | 7 (30) | 9 (30) | 15 (30) |
| PD | 14 (61) | 12 (40) | 25 (50) |
| Response rates, n (%) (95% CI) | |||
| CR+VGPR | 1 (4) (<1, 22) | 0 | 1 (2) (<1, 11) |
| ≥PR | 2 (9) (1, 28) | 8 (27) (12, 46) | 9 (18) (9, 31) |
| ≥MR | 2 (9) (1, 28) | 9 (30) (15, 49) | 10 (20) (10, 34) |
| Response . | Dose-escalation cohorts (n = 23) . | Expansion cohorts (n = 30*) . | Total (N = 50) . |
|---|---|---|---|
| Best response, n (%) | |||
| CR | 0 | 0 | 0 |
| PR | 2 (9) | 8 (27)† | 9 (18)† |
| VGPR | 1 (4) | 0 | 1 (2) |
| MR | 0 | 1 (3) | 1 (2) |
| SD | 7 (30) | 9 (30) | 15 (30) |
| PD | 14 (61) | 12 (40) | 25 (50) |
| Response rates, n (%) (95% CI) | |||
| CR+VGPR | 1 (4) (<1, 22) | 0 | 1 (2) (<1, 11) |
| ≥PR | 2 (9) (1, 28) | 8 (27) (12, 46) | 9 (18) (9, 31) |
| ≥MR | 2 (9) (1, 28) | 9 (30) (15, 49) | 10 (20) (10, 34) |
CR, complete response; PD, progressive disease; VPGR, very good partial response.
Includes 3 patients from the MTD dose-escalation cohort.
2 PRs unconfirmed; 1 PR unconfirmed but confirmed as MR. Responses were seen across the expansion cohorts, including 2, 3, 1, and 2 PRs in the relapsed and refractory, bortezomib-relapsed, PI-naive, and prior carfilzomib cohorts, respectively.
Ixazomib treatment duration and response. (A) Number of cycles on therapy and response to treatment in patients achieving stable disease or better; (B) individual patients’ best M-protein response to treatment, by treatment cohort.
Ixazomib treatment duration and response. (A) Number of cycles on therapy and response to treatment in patients achieving stable disease or better; (B) individual patients’ best M-protein response to treatment, by treatment cohort.
Pharmacokinetics
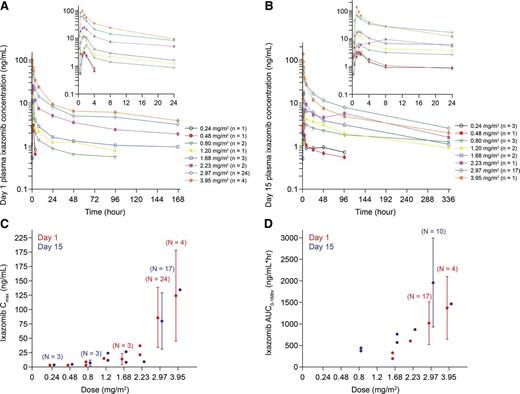
Ixazomib was rapidly absorbed resulting in a median time to plasma peak concentration of 1 hour (range, 0.5 to 8.0 hours). After multiple dosing, the terminal half-life was 3.6 to 11.3 days, and the accumulation ratio (day 15 area under the curve [AUC]0-168hr/day 1 AUC0-168hr) was 2.0 (supplemental Table 3). Dose-proportionality analysis, examining the relationship between AUC0-168hr and absolute dose in milligrams using linear regression, gave a calculated slope of the linear regression line (using log-transformed data) of 1.34 (95% confidence interval [CI], 0.29-2.40) on day 1 and 1.01 (95% CI, 0.46-1.55) on day 15. Both CIs contain 1.0, supporting the conclusion of dose proportionality in ixazomib exposure over the dose range of 0.8 to 3.95 mg/m2 (actual administered dose range 1.4-8.9 mg). The mean plasma concentration–time profiles on days 1 and 15 of cycle 1 are shown in Figure 4A-B, and the geometric mean day 1 and day 15 plasma peak concentration and AUC0–168hr are shown in Figure 4C-D, across the different dose levels. Pharmacokinetic data were similar across the expansion cohorts.
Ixazomib pharmacokinetic profiles in cycle 1, according to dose. Mean plasma day 1 (A) and day 15 (B) concentration–time profiles; (C-D) geometric mean (%CV) day 1 and day 15 plasma Cmax and AUC0–168hr. Cmax, peak concentration.
Ixazomib pharmacokinetic profiles in cycle 1, according to dose. Mean plasma day 1 (A) and day 15 (B) concentration–time profiles; (C-D) geometric mean (%CV) day 1 and day 15 plasma Cmax and AUC0–168hr. Cmax, peak concentration.
Discussion
The current study represents one of the first reports of clinical results with ixazomib, a novel orally bioavailable PI, in MM. In this trial, we have defined a tolerable and effective dose of oral ixazomib given weekly in this relapsed and/or refractory patient population. Overall, the drug was generally well tolerated, with most of the observed toxicities manageable with supportive care and dose reductions. Gastrointestinal and skin toxicities were among the most common drug-related AEs and there appeared to be a reduced risk of severe PN compared with bortezomib. Data from this phase 1 single-agent study included clinically meaningful responses in more than one-quarter of patients with relapsed/refractory MM treated at the MTD. Finally, detailed pharmacokinetic and studies undertaken in conjunction with the clinical trial demonstrated a long terminal half-life, supporting the use of a weekly dosing strategy.
Bortezomib has typically been administered twice-weekly until recently. Several studies have shown that it can be administered on a once-weekly schedule in combination regimens without significantly affecting the cumulative dose administered or the efficacy, but at the same time improving the toxicity profile by reducing the risk of PN.28,29 Given this background, oral ixazomib has been evaluated in phase 1 studies using both dosing schedules. In the current study, we determined the MTD of weekly ixazomib to be 2.97 mg/m2, which contrasts with the MTD of 2.0 mg/m2 determined for twice-weekly ixazomib.38 Moreover, less frequent dosing may favorably affect the toxicity profile, given the differences observed between the 2 phase 1 trials evaluating the weekly and twice-weekly dosing schedules. However, accommodating for cycle-length differences with the 2 schedules, the weekly planned doses of ixazomib were similar, at 2.25 and 2.67 mg/m2 for the weekly and twice-weekly schedules, respectively, resulting in the delivery of similar cumulative doses. The long terminal half-life of 3.6 to 11.3 days seen with repeated dosing in the current study supports weekly dosing. Additionally, the accumulation ratio was consistent with the weekly dosing schedule and observed terminal half-life.
The toxicity profile seen with oral ixazomib, whereas similar to bortezomib in certain aspects, is quite different in others. The most common drug-related toxicities in the present study, including DLTs, included gastrointestinal AEs and dermatologic toxicity. Nausea, vomiting, and diarrhea were among the most common toxicities with bortezomib in the phase 3 Assessment of Proteasome Inhibition for Extending Remissions (APEX) trial of bortezomib versus dexamethasone.17 Similarly, in the current study and in the twice-weekly dosing study of ixazomib,38 gastrointestinal toxicity was among the most common AEs. Fatigue, one of the most common toxicities observed in the current study, was also reported commonly with bortezomib.16,17 Skin rash (single Medical Dictionary for Regulatory Activities [MedDRA] preferred term) was seen in 18% of patients with bortezomib in APEX, with 1% grade 3 or higher events.17 The overall rate of drug-related skin toxicity (all AEs within the MedDRA system organ class) was 22% in the current study, including 3% grade 3 events: 1 of the 3 DLTs was grade 3 skin rash, albeit at the highest ixazomib dose level (above the MTD), and 1 patient in the MTD expansion cohorts had grade 3 papular rash. Importantly, rash was reported as a DLT in the parallel trial of twice-weekly ixazomib38 as well as in a phase 1/2 study of weekly ixazomib in combination with lenalidomide and dexamethasone, for which rash is an overlapping toxicity with lenalidomide.39 Given the relatively low overall frequency of this AE, it is possible that the development of rash is a dose-dependent effect.
Two other AEs seen in the current study require particular mention in the context of experience with bortezomib. Thrombocytopenia, as with bortezomib,16,17 was common. However, this appeared to be a drug-related transient effect, with patients’ platelet counts showing a cyclical pattern, dropping after ixazomib dosing and returning toward baseline levels before the next cycle. Only 5 patients required platelet transfusions. This is reminiscent of the pattern seen with bortezomib.17,40,41 In support of ixazomib showing improved aspects of its safety profile compared with bortezomib, we observed a very low frequency of severe PN. Although the overall rate of drug-related PN was 20%, with a rate of 32% in the MTD expansion cohorts (compared with 31% to 36% with single-agent bortezomib in the relapsed/refractory setting16,17 ), only 1 patient had grade 3 PN. This compares favorably with PN rates reported with subcutaneous bortezomib26 and newer PIs such as carfilzomib.34,35,42
Overall, the drug was generally well tolerated, with most of the observed toxicities manageable with supportive care and dose delays or reductions. No cumulative toxicities have been observed, including no cumulative hematologic toxicity, indicating the potential feasibility of long-term treatment. Given that this was a phase 1 study in a heavily pretreated patient population, the majority of patients received less than 4 cycles of therapy; the tolerability of long-term oral ixazomib administration needs further study and all aspects of the safety profile need confirming in future trials. In the current study, nearly 20% of patients received more than 6 cycles and 10% received 11 to 12 cycles. In addition, nearly 90% of the planned dose was delivered over the cycles of therapy, again highlighting the tolerability of the drug.
Finally, we saw encouraging antimyeloma activity in this phase 1 trial with dose expansion cohorts based on varying prior exposure to available drugs in a patient population who had received a median of 6 prior regimens over the course of a median of almost 5 years from their initial diagnosis; 85%, 97%, 53%, and 15% of patients had received prior therapy with bortezomib, lenalidomide, thalidomide, and carfilzomib, respectively. Nearly three-quarters of patients were refractory to their last prior therapy, including 18% who were bortezomib-refractory and 38% who were lenalidomide/thalidomide-refractory. Among those treated at the MTD, we saw PR or better in 27% of patients. This appears comparable with the rate seen with twice-weekly, single-agent bortezomib in heavily pretreated MM patients in the phase 2 Study of Uncontrolled Myeloma Management with proteasome Inhibition Therapy (27%)16 and with single-agent carfilzomib in MM patients who had previously received bortezomib and thalidomide/lenalidomide in the phase 2 PX-171-003A study (24%).34 Unlike the bortezomib trials, we did not see any complete responses, which was not a surprising result given that the patients in those earlier trials were not previously exposed to the same novel agents as the current cohorts. The responses seen in the present study have been durable in many of the patients, with 5 of the responding patients staying on therapy for more than 8 cycles.
In conclusion, this phase 1 study has identified the MTD of weekly single-agent ixazomib in the setting of relapsed/refractory MM and provided preliminary data on the safety and tolerability of oral ixazomib in this patient population. The anti-MM activity seen here appears comparable to that seen with bortezomib in the “pre-PI era” as well as to that seen with the newer PI carfilzomib in a similar patient population. The ease of oral administration and the weekly schedule provide a convenient potential approach to MM therapy. Trials examining various combination regimens involving ixazomib are under way in MM; of note, preliminary phase 1/2 data have been reported with ixazomib in combination with lenalidomide plus dexamethasone in the newly diagnosed setting.39 Double-blind phase 3 studies of ixazomib or placebo in combination with lenalidomide plus dexamethasone are ongoing in the relapsed and/or refractory (NCT01564537) and newly diagnosed transplant-ineligible (NCT01850524) settings.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients included in this study and their families, all physicians, research nurses, study coordinators, and research staff participating in this study. The authors also acknowledge Steve Hill, a medical writer with FireKite, for writing support during the development of this manuscript, which was funded by Millennium: The Takeda Oncology Company.
The clinical trial was sponsored by Millennium: The Takeda Oncology Company.
Authorship
Contribution: S.K.K., W.I.B., T.M.Z., C.B.R., J.R.B., D.B., A.-M.H., N.G., Y.S., and R.N. designed the research; S.K.K., W.I.B., T.M.Z., C.B.R., J.R.B., D.B., N.G., Y.S., and R.N. performed the research; S.K.K., W.I.B., T.M.Z., C.B.R., J.R.B., D.B., N.G., J.Y., Y.S., and R.N. collected data; N.G., J.Y., and Y.S. performed statistical analysis; S.K.K., W.I.B., T.M.Z., C.B.R., J.R.B., D.B., A.-M.H., N.G., Y.S., and R.N. analyzed and interpreted data; S.K.K., N.G., and Y.S. wrote the manuscript; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: S.K.K.: consultant, Merck, Celgene, Millennium Pharmaceuticals, Inc., Onyx; research funding, Millennium Pharmaceuticals, Inc., Celgene, Novartis, Genzyme, Onyx, Cephalon; W.I.B.: consultant, honoraria, Celgene, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals; speakers bureau, Celgene; research funding, Celgene, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals, Array, AstraZeneca, Genentech; T.M.Z.: expert witness, Novartis; honoraria, speakers bureau, Celgene, Millennium Pharmaceuticals, Inc.; C.B.R.: institutional research funding, Celgene, Millennium Pharmaceuticals, Inc., Novartis; J.R.B.: consultant, honoraria, speakers bureau, research funding, Novartis, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals, Celgene, Medtronic; research funding, Merck, Genentech; D.B., A.-M.H., N.G., J.Y., and Y.S.: employment, Takeda Pharmaceuticals International Co.; and R.N.: consultant, speakers bureau, research funding, Celgene, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals.
Correspondence: Shaji K. Kumar, Division of Hematology, Mayo Clinic, Rochester, MN 55906; e-mail: kumar.shaji@mayo.edu.