In this issue of Blood, Mensen et al provide a comprehensive evaluation of B-cell development in adult patients who underwent allogeneic hematopoietic transplantation.1
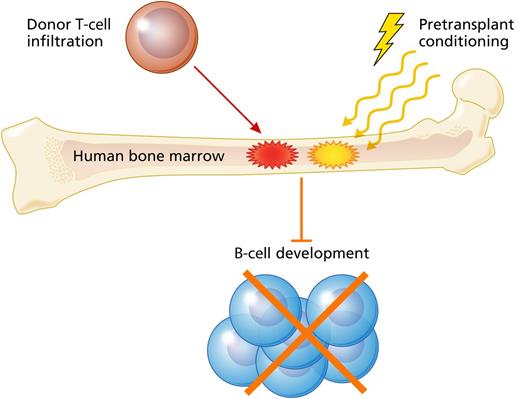
Mensen et al present data indicating that GVHD, donor T-cell infiltration in recipient marrow, and high intensity pretransplant conditioning were associated with impaired B-cell reconstitution in adult patients who underwent allogeneic hematopoietic transplantation for treatment of acute leukemia. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mensen et al present data indicating that GVHD, donor T-cell infiltration in recipient marrow, and high intensity pretransplant conditioning were associated with impaired B-cell reconstitution in adult patients who underwent allogeneic hematopoietic transplantation for treatment of acute leukemia. Professional illustration by Patrick Lane, ScEYEnce Studios.
To paraphrase Oscar Wilde, one could say that clinical transplantation imitates mouse models. This has led to extensive knowledge of graft-versus-host disease (GVHD) pathophysiology, although it remains a serious complication after transplant. The pervasive nature of acute GVHD is becoming increasingly apparent, as the list of potential targets has expanded beyond the skin, liver, and gastrointestinal tract to include the lungs, central nervous system, and thymus.2-4 Myelosuppression has long been appreciated clinically in patients experiencing GVHD, and recent data from mouse models have indicated that the bone marrow itself may be a target of acute GVHD, leading to impaired hematopoiesis due to damage of the bone marrow niche.5,6 Similar to the thymus’s role in T-cell development, the bone marrow is an important site of immune reconstitution after transplant, in this case for B-cell development. Although it is appreciated that B-cell recovery can be impaired in patients with GVHD, little is known about bone marrow involvement in clinical GVHD and the relationship this may have with B-cell reconstitution.
Armed with knowledge gained from mouse models, Mensen et al set out to characterize B-cell reconstitution after allogeneic transplant in patients. Using mobilized peripheral blood transplantation into allogeneic recipients with acute leukemia as their model system, the authors performed an encyclopedic assessment of the kinetics of reconstitution for various B-cell subsets. They found that delayed B-cell reconstitution correlated with development of GVHD, as well as with high intensity conditioning (see figure). This connection between GVHD and B-cell deficiency is consistent with 2 other recent studies.7,8 However, the latter point is particularly interesting given reports indicating the importance of pretransplant conditioning for B-cell reconstitution in pediatric patients with severe combined immunodeficiency disease.9,10
After characterizing the B-cell recovery deficit in GVHD, the authors then performed further evaluation of the site of B-cell development, the bone marrow niche, determining that impairment of B-cell reconstitution correlated with the degree of T-cell infiltration in the bone marrow. Furthermore, in a subset of patients, the authors were able to perform chimerism analysis on the bone marrow T cells, confirming that they were indeed of donor origin. This valuable insight indicated that the infiltrating T cells were not residual host T cells impairing donor hematopoiesis and B-cell development by mediating occult subclinical host vs graft rejection responses.
The work of Mensen et al makes a compelling association between GVHD, T-cell infiltration of the marrow, and impaired B-cell recovery. Although the role of B cells in GVHD has become a major point of interest in the field recently, as evidenced by the scientific session on B cells in GVHD at the 2013 ASH Annual Meeting, here the authors focus on how GVHD regulates B cells. Their findings raise several important questions. It is not clear how much of the B-cell deficiency they identified was due to GVHD directly as opposed to immunosuppressive GVHD treatment. Additionally, if GVHD was directly responsible for delayed B-cell recovery, it is possible that the T-cell marrow infiltration is a nonspecific finding in GVHD patients and not causative for B-cell deficiency. These are important areas for future research.
Another critical issue is the significance of T-cell marrow infiltration and B-cell development for transplant outcome. Although the authors showed that impaired B-cell reconstitution after transplant was associated with reduced B-cell function ex vivo, it is not clear from their findings that these patients necessarily have worse outcomes. If so, prospective studies may be necessary to establish definitions for bone marrow GVHD, possibly based on the degree of T-cell marrow infiltration and expression of κ-deleting recombination excision circles, and evaluate potential interventions. However, it is interesting to note that systemic GVHD correlated with increased marrow T-cell infiltration and that GVHD is often associated with reduced malignant relapse. One could thus be tempted to speculate that bone marrow GVHD may be closely related to graft-versus-leukemia (GVL) responses and the curative potential of the transplant.
Future work should explore the potential relationship between T-cell infiltration of the marrow and prevention of leukemia relapse. Furthermore, for experimental transplanters trying to separate GVHD from GVL in mice, where it is challenging to model transplants in leukemia-bearing hosts, it may be more efficient to study pathophysiology distinguishing epithelial organ GVHD from that in the marrow. Nonetheless, it should be noted how closely the findings of Mensen et al have mirrored the findings in mouse models of GVHD for T-cell infiltration of the marrow, damage to the osteoblast niche, and impairment in B-cell development.5,6 The authors have made a significant step forward in our understanding of B-cell development after transplant and its impairment in clinical GVHD.
Conflict-of-interest disclosure: The authors declare no competing financial interests.