In this issue of Blood, Deng et al have clarified the molecular mechanism critical for the angiopoietin-like 2 (Angptl2) binding to its receptor leukocyte immunoglobulin-like receptor B2 (LILRB2), providing a theoretical basis for Angptl2-driven ex vivo expansion of human hematopoietic stem cells (HSCs).1
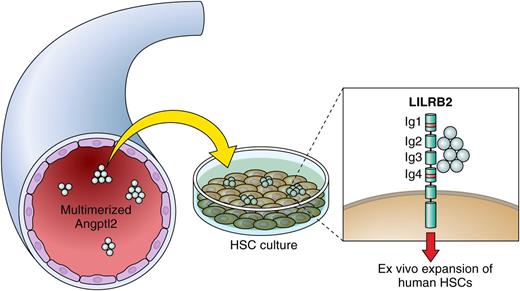
Model of the action of Angptl2 on HSCs. Multimerized Angptl2, detected in peripheral blood, binds 2 motifs of LILRB2, Y96 in Ig1 and G392 in Ig4, resulting in ex vivo expansion of human HSCs. Professional illustration by Patrick Lane, ScEYEnce Studios.
Model of the action of Angptl2 on HSCs. Multimerized Angptl2, detected in peripheral blood, binds 2 motifs of LILRB2, Y96 in Ig1 and G392 in Ig4, resulting in ex vivo expansion of human HSCs. Professional illustration by Patrick Lane, ScEYEnce Studios.
The regulation and manipulation of HSCs are important issues in clinical practice, particularly in the treatment of hematologic malignancies. Transplantation of HSCs is critical for patients’ survival when potent chemotherapy or systemic irradiation, which eliminates the normal hematopoietic system, is required to clear malignant cells. However, it is often difficult to get a sufficient number of HSCs for this purpose. In this light, ex vivo expansion of HSCs is a fascinating strategy for overcoming this issue. A number of molecular targets or biological reagents have been identified to increase the number of transplantable HSCs in vitro or in vivo,2 although their clinical utility has yet to be defined. In this line, Zhang and colleagues have been exploring the potential of using members of the Angptl protein families, in particular Angptl2, as candidate HSC expanders.3,4
Angiopoietin signaling through the Tie-2 receptor is widely known to play a key role in vascular development in mammals.5 All members of the angiopoietin family possess 2 characteristic structures: a coiled-coil domain (CCD), which likely contributes to oligomerization, and a fibrinogen-like domain (FLD), which is thought to be the receptor binding domain. Several other genes encode proteins containing both the CCD and the FLD. Although these factors were initially predicted to function as ligands for Tie receptors, they were found to bind neither Tie1 nor Tie2. Therefore, they are designated (Angptls) and have long been considered orphan ligands.6 Among Angptls, Angptl2, first identified by Koh and colleagues,7 is particularly remarkable for its versatile function in angiogenesis, metabolism, inflammation, and possibly tissue regeneration.6-9 In 2006, Zhang and colleagues reported that the HSC-supportive mouse fetal liver CD3+ cells highly express Angptl2. Notably, they observed 10-day culture of murine HSCs with purified Angptl2 confers 24-fold net expansion of long-term HSCs by reconstitution analysis.3 Moreover, they recently discovered that several Angptls including Angptl2 bind and activate the immune inhibitory receptor human LILRB2 and its mouse ortholog paired immunoglobulin-like receptor.4
Currently, one of the most important questions is how Angptl2 binds and activates LILRB2. In the present paper by Deng et al,1 the authors addressed this issue. Oligomerization of Angptl2 via the CCD is required for activation of the LILRB2 signaling. Also, 2 motifs of LILRB2, Y96 in immunoglobulin 1 (Ig1) and G392 in Ig4, were necessary for the receptor to be bound and activated by Angptl2. Considering future development, because Angptls are large glycosylated proteins that are readily degraded and form aggregates, molecules with enhanced stability and higher activities that mimic the effects of Angptl2 would lead to the development of a more efficient HSC expansion system. Based on this idea, the authors found immobilized antibodies against LILRB2 mimic Angptl2-stimulated receptor signaling and support ex vivo expansion or maintenance of human cord blood HSCs.
Of further interest is the significance of the Angptl2 oligomerization in tissues outside hematopoietic organs. This point is particularly important, as Angptl2 seems to be acting as an integrin ligand, but not via LILRB2, outside the hematopoietic system.6,8 Overall, a series of studies by Zhang and colleagues clearly indicate that Angptl2/LILRB2 signaling is one of the most promising targets for ex vivo expansion of human HSCs. Application of immobilized anti-LILRB2 antibodies will be practically and therapeutically suitable for this purpose.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal