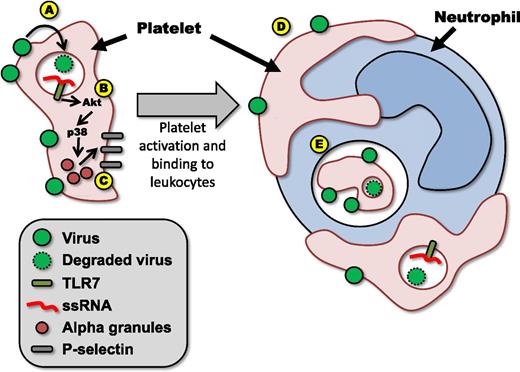
(A) Virus is bound by platelets and internalized. (B) Viral particles are degraded, releasing ssRNA, which binds TLR7, and initiating signaling through Akt and p38–mitogen-activated protein kinase (MAPK). (C) Platelet activation results in morphological changes and release of α-granules, leading to increased expression of molecules such as P-selecting on the platelet surface. (D) Activated platelets bind to leukocytes in the circulation such as neutrophils. (E) Bound platelets (or fragments derived from bound platelets) are then phagocytosed by the neutrophil.
(A) Virus is bound by platelets and internalized. (B) Viral particles are degraded, releasing ssRNA, which binds TLR7, and initiating signaling through Akt and p38–mitogen-activated protein kinase (MAPK). (C) Platelet activation results in morphological changes and release of α-granules, leading to increased expression of molecules such as P-selecting on the platelet surface. (D) Activated platelets bind to leukocytes in the circulation such as neutrophils. (E) Bound platelets (or fragments derived from bound platelets) are then phagocytosed by the neutrophil.
It has long been known that severe viral infection often results in thrombocytopenia, although the specific mechanism(s) leading to this reduction in circulating platelets has remained elusive.2 Whereas some have suggested the loss of platelets is due to lysis and reduced production, others have provided evidence that platelets become sequestered within the microvasculature of organs such as the liver.3 Furthermore, it had been unknown if platelets were directly activated by the virus or if platelets were simply responding secondarily to the activation of other immune components. Previous studies demonstrated that platelets express functional TLRs and were able to directly detect and respond to bacterially derived pathogen associated molecular patterns (PAMPs) such as lipopolysaccharide and CpG DNA.4,5 Although these studies provided proof-of-concept that platelets were able to recognize pathogens, they failed to address questions surrounding the interaction between platelets and viruses.
In the current study, the authors demonstrate that in blood samples isolated from healthy human donors, platelets had measurable TRL7 protein expression.1 TLR7 is a pattern recognition receptor that specifically recognizes single-stranded RNA (ssRNA), a classic viral PAMP, contained within endosomes. Moreover, Koupenova et al1 were able to show, using either the TLR7 agonist Loxo or EMCV, that platelet TLR7 is functional, and ligand binding by this receptor results in platelet activation and degranulation. These studies represent a critical advancement in the field, as they clearly demonstrate that viruses are able to directly activate platelets to initiate a host response to infection. Interestingly, when platelet-derived mRNA from a large cohort of patients was examined, only ∼60% of samples possessed transcripts for TLR7. This finding was particularly intriguing, as it suggests that platelets are equipped with their array of TLR7 molecules during development, possibly as part of the megakaryocyte, and although some platelets possess TLR7 mRNA, it is not required for the expression of functional TLR7 protein.
Although these experiments demonstrated that viruses are able to activate TLR7 on platelets, one must remember that TLR7 is present within endosomes and as such requires internalization of the virus to initiate signaling. Electron microscopy revealed both extensive binding of EMCV viral particles to the surface of the platelet (presumably due to the expression of sialic acid on the surface of the platelet) and a marked accumulation of viral particles within the platelet (possibly within primary lysosomes). Upon activation via TLR7, platelets undergo rapid and dramatic changes; using a series of well-devised experiments, Koupenova et al1 effectively elucidate the mechanisms underlying these changes (see figure). Rapid phosphorylation of Akt and p38-MAPK leads to fusion and release of platelet α-granules. This process results in expression of molecules such as CD40L and P-selectin on the surface of the platelet, allowing the platelet to adhere to leukocytes forming heterotypic aggregates (HAGs). During this activation, platelets undergo morphological changes, displaying pronounced membrane ruffling and extension of long, thin pseudopodia. These morphological changes allow the platelet to maintain intimate contact with the surface of the leukocyte. Over time, leukocytes, particularly granulocytes, are observed to internalize these adherent platelets (or possibly fragments derived from these platelets). Extending these findings from an in vitro model of platelet stimulation to a model of in vivo viral infection, the authors show that infection with EMCV leads to TLR7-mediated thrombocytopenia in mice and induces the generation of circulating HAGs and, importantly, that platelet TLR7 is essential for animal survival in this model.
Although platelets are classically considered to be central mediators of hemostasis, there is increasing evidence to show substantial overlap between thrombosis and immunity.6 Many aspects of coagulation appear to play a role in limiting pathogen dissemination, enhancing immune activation, or mediating direct pathogen killing. Interestingly, this current work appears to demonstrate delineation between virus-mediated platelet activation and thrombosis. Although virally activated platelets have increased adherence to leukocytes and collagen (a molecule frequently associated with the initiation of coagulation in response to the exposure of the subendothelium following vascular damage), TLR7-mediated platelet activation failed to induce platelet-platelet aggregation or thrombosis. This finding is similar to that observed in a model of intravascular infection with Bacillus cereus7 and suggests it may be possible to functionally “uncouple” the hemostatic and immune functions of platelets.
Perhaps the most important finding in the current work is that platelet TLR7 contributes to host survival following viral infection.1 Mice deficient for TLR7 or depleted of platelets succumbed to the virus more rapidly than wild-type mice. The authors then went on to demonstrate that protection could be conferred to TLR7-deficient mice following transfusion of wild-type platelets. This surprising finding raises one critically important question: How? Is it that platelets are simply able to capture and sequester viral particles, targeting them for destruction through granulocyte phagocytosis? Or do platelets play a more active role in helping drive host antiviral immunity? Platelets have been shown to modulate cellular adhesion within the vasculature8 and enhance leukocyte activation through the release of soluble mediators such as sCD40L,9 potentially helping to regulate the host immune response to virus. More recently, platelet adherence to neutrophils has been shown to trigger the release of neutrophil extracellular traps within the vasculature.10 These structures, previously associated with antibacterial and antifungal immunity, have also been shown to protect from viral infection and as such potentially represent an additional mechanism by which platelets can contribute to antiviral immunity.3
As studies into platelet-mediated immunity advance, it is unlikely any one single mechanism will account for the protective functions of platelets, but rather it will likely be a battery of overlapping immune processes that allow the platelet to respond to a diverse array of potential pathogens. It is this adaptability that places the platelet as a key player in host immunity to viruses.
Conflict-of-interest disclosure: The author declares no competing finical interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal