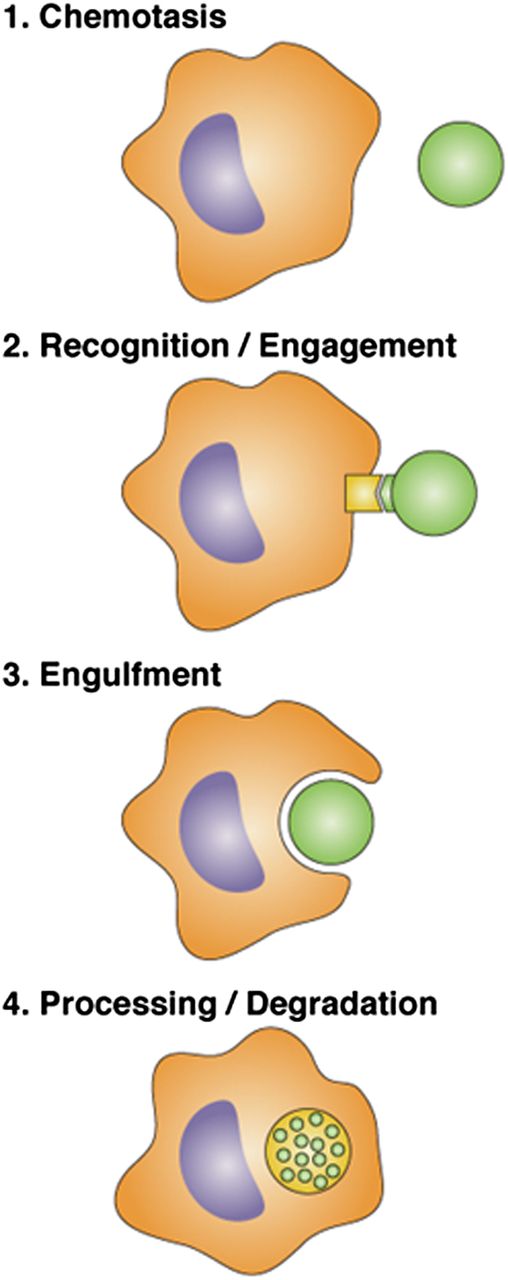
Steps at which plasmin(ogen) can regulate phagocytosis by macrophages. (1) “Find me” signals are released from “prey bodies,” resulting in chemotaxis by macrophages. Previous studies have established that plasminogen is required for recruitment of macrophages to sites of inflammation.2 Das et al show that (2) there is some effect of plasmin on prey bodies in the ability to be recognized by macrophages; (3) the primary effect of plasmin is on macrophages so that engulfment occurs more efficiently; and (4) expression of genes that regulate phagosome maturation and processing is down-regulated in plasminogen deficient compared with wild-type mice.
Steps at which plasmin(ogen) can regulate phagocytosis by macrophages. (1) “Find me” signals are released from “prey bodies,” resulting in chemotaxis by macrophages. Previous studies have established that plasminogen is required for recruitment of macrophages to sites of inflammation.2 Das et al show that (2) there is some effect of plasmin on prey bodies in the ability to be recognized by macrophages; (3) the primary effect of plasmin is on macrophages so that engulfment occurs more efficiently; and (4) expression of genes that regulate phagosome maturation and processing is down-regulated in plasminogen deficient compared with wild-type mice.
Plasminogen is well known as the zymogen form of plasmin, the primary fibrinolytic enzyme, and as such plays a vital role in protecting the host from thrombotic events. Studies conducted in plasminogen-deficient mice conducted for more than a decade have demonstrated a vital role of plasminogen/plasmin as a facilitator of leukocyte recruitment, in general, and macrophage recruitment, in particular, to sites of inflammation. Thus, in murine models of atherosclerosis, arthritis, asthma, and wound healing, macrophage infiltration is blunted in plasminogen-deficient mice.2 There are also more limited lines of evidence emerging that plasminogen can affect macrophage functions beyond altering the migratory properties of the cells. For example, plasminogen has been shown to stimulate signaling pathways in macrophages,3 to regulate macrophage apoptosis,4 and to enhance uptake of oxidized lipoproteins leading to foam cell formation.5 None of these prior studies provided a foundation for anticipating that plasminogen would exert a profound effect on the phagocytic function of macrophages, a function that is necessary to protect the host against invading pathogens and to rid the host of unwanted debris.
Macrophage phagocytic function involves several steps (see figure). The initial step requires macrophage recruitment to inflammatory sites, which has previously been shown to be markedly impaired in plasminogen deficient mice.2 (Das et al used both in vitro and in vivo models of macrophage phagocytosis that did not depend on macrophage recruitment to focus on the role of plasminogen on subsequent steps in phagocytosis.) The second step involves recognition and engagement of prey by macrophages. Treatment of prey with plasmin inhibitors resulted in a 30% decrease in phagocytosis.1 The major effect of plasmin was, however, on macrophages and macrophage engulfment of prey (step 3).1 Treatment of macrophages with plasminogen markedly stimulated uptake of prey, and plasmin activity and protein synthesis were required. Furthermore, clearance of apoptotic cells from the spleen and clearance of apoptotic thymocytes from the peritoneum were markedly reduced in plasminogen-deficient mice, as was phagocytosis by liver Kupffer cells in a hemolytic anemia model.1
Das and colleagues perform gene array studies and make the remarkable observation that plasminogen modulates expression of major genes involved in phagocytosis, including phagocytic receptors, recognition and engulfment molecules, phagosome maturation genes, phagosome processing genes, and signal transduction molecules. Thus, plasmin may regulate all of the steps in phagocytosis by stimulating cellular signaling.
This study provides a strong rationale for future studies to investigate the role of specific molecules in plasminogen-dependent phagocytosis by macrophages. Das and colleagues demonstrate that the effects of plasminogen were modulated by a direct interaction of plasminogen with cells. As several distinct plasminogen receptors are present on the surfaces of macrophages,6 future studies are warranted to elucidate the roles of specific plasminogen receptors in plasmin-dependent signaling during the different steps in phagocytosis and whether these or other macrophage cell surface proteins are specific targets of plasmin. Subsequently, the specific signaling pathways induced by plasmin that lead to up-regulation of phagocytosis genes can be identified. There may also be additional mechanisms by which plasminogen plays a role in phagocytosis by mediating prey recognition. For example, certain plasminogen receptors that are tethered to cells by phosphatidyl serines7 may bind plasminogen on apoptotic prey cells that can then be recognized by a transmembrane plasminogen receptor8 on macrophages.
This novel function of plasminogen has important clinical implications. As pointed out by Das et al, deficiency of plasminogen or the presence of antibodies to plasminogen receptors is correlated with several disease states with an autoimmune and/or inflammatory component. Furthermore, Das et al demonstrate that the effects of plasminogen on phagocytosis are blocked by tranexamic acid, a lysine mimetic that interacts with plasminogen to block plasmingen binding to cells and to fibrin. Tranexamic acid is used therapeutically to inhibit fibrinolysis and hemorrhage in several conditions including surgery, angioedema, menorrhagia, and dental procedures in patients with genetic deficiencies of coagulation factors or of plasmin inhibitors. Thus, treatment with tranexamic acid and other fibrinolysis inhibitors may have important clinical effects that are yet to be elucidated. Finally, this novel study by Das et al points the way for consideration of plasminogen and plasminogen receptors as potential therapeutic targets in patients with defective innate immunity.
Conflict-of-interest disclosure: The authors declare no competing financial interests.