In this issue of Blood, van Eggermond et al demonstrate that Hodgkin lymphoma survivors who develop a second malignancy have increased risk of developing yet a third malignancy.1
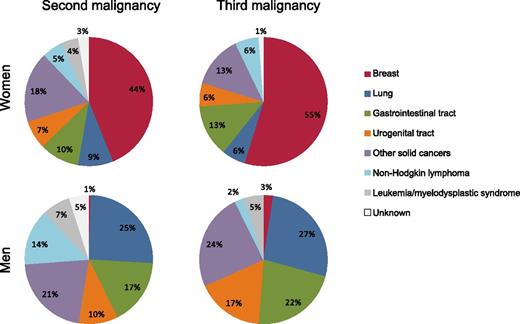
Distribution of second and third malignancies, by type and gender, among 3122 Dutch patients who were originally diagnosed with HL by age 50 during 1965 to 1995, survived ≥5 years, and were followed through 2012.1
Distribution of second and third malignancies, by type and gender, among 3122 Dutch patients who were originally diagnosed with HL by age 50 during 1965 to 1995, survived ≥5 years, and were followed through 2012.1
With major advances in treatment over the last several decades, Hodgkin lymphoma (HL) has become one of the most curable malignancies, with a 5-year relative survival following HL diagnosis now exceeding 85%.2 However, HL survivors also have some of the highest risks for developing second cancers, which are a major cause of morbidity and mortality.3
van Eggermond et al reveal that consideration of “second” cancer risks provides an incomplete picture of the long-term health of HL survivors because a proportion will go on to develop a third or even fourth malignancy. They systematically collected data on subsequent malignancies in >3000 Dutch patients, who were originally diagnosed with HL by age 50 during 1965 to 1995, survived at least 5 years, and were followed through 2012. Such long-term follow-up is critical, because the median time from HL to second malignancy was 19 years and from second to third malignancy was another 4 years.
The persistently increased cancer risk for decades after HL diagnosis and the distribution of second and third malignancies by type (see figure) focuses our attention on several key issues: the major role of HL treatments—particularly radiotherapy—in subsequent malignancies, the question of whether some survivors may be more susceptible to subsequent malignancies than others, and how these results inform HL treatment and long-term follow-up practices.
Radiotherapy clearly is the dominant contributor to both second and third malignancies. Female breast, lung, and gastrointestinal tract cancers were the most frequently observed malignancies after HL in the Dutch cohort, and previous studies have demonstrated that risks for these malignancies are strongly related to the radiation dose these organs receive during HL treatment.4-6 Ninety percent of patients in the Dutch cohort received radiotherapy, typically with extended fields because the majority of patients were treated before the introduction of involved field irradiation around 1990. The impact of supra-diaphragmatic, extended field radiotherapy on subsequent malignancy risk is particularly evident from the substantial proportion of subsequent breast cancers among female HL survivors (44% of second malignancies and 55% of third malignancies) and subsequent lung cancers among male HL survivors (25% of second malignancies and 27% of third malignancies). The impact of changes in radiotherapy for HL (eg, lower doses, smaller field sizes, novel techniques) on subsequent malignancy risk remains unclear because most radiation-related malignancies occur 1 to 2 decades or more after exposure.7
HL chemotherapy plays a smaller role in subsequent malignancy risks. In the Dutch cohort, about 40% of patients received chemotherapy, typically a MOPP (mechlorethamine, vincristine, procarbazine, prednisone) or MOPP-like regimen. Previous studies have demonstrated increased risks of lung cancer with MOPP4 and a synergistic effect of procarbazine-containing chemotherapy and radiotherapy on stomach cancer,6 whereas breast cancer risks are reduced with cytotoxic chemotherapy due to its impact on ovarian function.5 Little is known about the spectrum of subsequent malignancy risks with current chemotherapy regimens such as ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine). Although the striking chemotherapy-related risks of acute myeloid leukemia are well established, these malignancies tend to occur relatively quickly following exposure, and thus they accounted for few second or third malignancies in the Dutch cohort, which was restricted to 5-year survivors.
Are some survivors particularly susceptible to developing subsequent malignancies? The observation of van Eggermond et al that patients who develop a second malignancy have higher risk for developing third and additional malignancies could merely reflect differences in treatment exposures, which were not accounted for in the analysis.1 However, they did report higher subsequent malignancy risks for HL survivors treated at a younger age. One explanation may be increased radiosensitivity of developing tissues, particularly the breast. Alternatively, host susceptibility such as inherited genetic variation or HL-related immune dysfunction may contribute, independently or as a modifier of treatment-related risks. Indeed, a small genome-wide association study identified a susceptibility locus at chromosome 6q21 related to second cancers among HL survivors treated with radiotherapy.8 Those results support the importance of larger-scale studies of genetic susceptibility to subsequent malignancies, such as ongoing efforts in the Childhood Cancer Survivor Study. Additional investigation also is warranted to understand whether underlying immune dysfunction may predispose certain HL survivors to developing additional immune-related malignancies,9 which could explain excesses of non-Hodgkin lymphoma and, to a lesser extent, melanoma, because neither of these malignancies has been clearly related to radiation or chemotherapy exposures.1,3
What is the impact of this research on long-term follow-up practices for HL survivors? Current screening protocols target surveillance based on survivors’ treatment exposures. Breast cancer screening following chest radiotherapy is the most accepted. However, the risks and benefits of such screening for specific groups of survivors are not well understood, and screening for other malignancies such as lung or gastrointestinal tract cancers is even less established. The data from the Dutch cohort indicate that subsequent malignancy risks persist, and may even be higher, after the diagnosis of a second malignancy. Thus, survivors of second malignancies could realize even greater benefits from increased surveillance. These results are consistent with a previous report from the Childhood Cancer Survivor Study demonstrating particularly high subsequent malignancy risks among survivors who developed non-melanoma skin cancer.10 Unfortunately, cancer registry-based studies such as the current Dutch study do not systematically ascertain the occurrence of non-melanoma skin cancer and thus cannot confirm that finding. Plausibly, a non-neoplastic adverse outcome also could serve as a marker for identifying patients at high risk of developing subsequent malignancies if the outcomes share an underlying pathogenesis (eg, skin erythema or subcutaneous fibrosis, which could reflect underlying susceptibility to radiation-induced tissue damage). Future research should consider a range of adverse outcomes to further explore this possibility.
Because of their favorable prognosis, frequent diagnosis at a young age, and common receipt of cytotoxic chemotherapy and/or radiotherapy, HL survivors have long been harbingers of the late effects of treatments that also may affect other cancer survivors. The data from van Eggermond et al are a valuable addition to the sparse literature on the occurrence of multiple subsequent neoplasms, and their findings remind us of the importance of continued efforts to reduce the late effects of treatment so that cancer survivors are not faced with double—or triple—jeopardy.
Conflict-of-interest disclosure: The author declares no competing financial interests.