Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 4004.
Disclosures
The authors, Associate Editor David Garcia, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe indications for use of hydroxyurea in adults with sickle cell anemia.
Describe indications for use of hydroxyurea in children with sickle cell anemia.
Describe indications for use of hydroxyurea in patients with HbSβ+-thalassemia.
Release date: December 18, 2014; Expiration date: December 18, 2015
Case presentation
What is the evidence to start hydroxyurea in the following cases?
Case 1: A 29-year-old man with HbSS was hospitalized for 2 weeks with acute chest syndrome, during which time he underwent red blood cell exchange transfusion. He is now 1 month post–hospital discharge with infrequent pain, which responds to nonsteroidal anti-inflammatory drugs.
Case 2: A 5-year-old girl with HbSS is being seen for a routine follow-up visit. She has never had acute chest syndrome or other sickle cell–related complications. She currently has minimal pain and has not had any emergency room visits over the past year.
Case 3: A 22-year-old woman with HbSβ+-thalassemia has frequent painful episodes and was hospitalized for the fourth time in the last 14 months.
Introduction
Patients with sickle cell disease (SCD; defined as homozygous hemoglobin [Hb] S and compound heterozygotes, eg, HbSC) have hemolytic anemia and vaso-occlusion that result in pain, organ injury, and premature mortality. No specific therapy was available for these patients until the 1970s, when 2 studies recognized that patients with increased red blood cell fetal hemoglobin (HbF) levels had fewer adverse clinical events.1,2 In 1984, pharmacologic induction of HbF by hydroxyurea was documented in 2 adults with sickle cell anemia (SCA; defined in this review as HbSS or Sβ0-thalassemia).3 Subsequent prospective studies verified the efficacy and tolerability of hydroxyurea,4,5 leading to a placebo-controlled, randomized controlled trial (RCT) that demonstrated the efficacy of hydroxyurea in reducing painful vaso-occlusive crises (VOC) and acute chest syndrome (ACS) in adults with SCA.6 The US Food and Drug Administration approved hydroxyurea in 1998 for the treatment of adults with SCA with recurrent moderate to severe painful crises. In 2007, the European Medicines Agency authorized hydroxyurea for recurrent VOC and ACS in pediatric and adult patients with sickle cell syndromes.7 In 2008, the Agency for Healthcare Research and Quality (AHRQ) published a comprehensive systematic review and a National Institutes of Health Consensus Development Conference was held on hydroxyurea treatment of SCD.8,9 Our current objective is to analyze the current literature and provide evidence-based recommendations for hydroxyurea therapy in SCD.
Methods
Data sources and searches
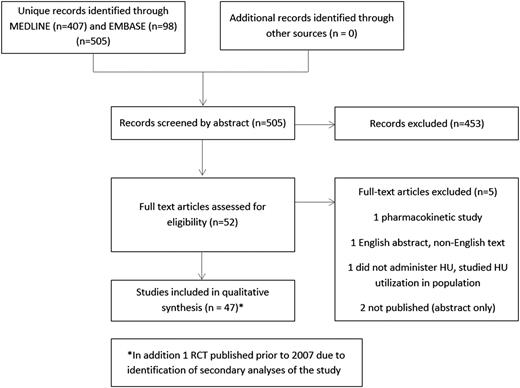
The 2008 AHRQ review conducted a search of MEDLINE, EMBASE, TOXLine, and CINAHL through June 2007. In the current study, we performed a MEDLINE and EMBASE search involving hydroxyurea treatment of humans with SCD from January 2007 through December 2013. The search included MeSH terms sickle cell, hydroxyurea, and hydroxycarbamide (see Figure 1 for our search strategy). We reviewed the references of systematic reviews and pertinent articles to identify additional studies.
Study selection, data extraction, and quality assessment
Study selection was done independently in duplicate by 2 investigators. Titles and abstracts of citations identified were reviewed for inclusion. Eligibility criteria were adapted from the AHRQ review.8 Articles were included if they were peer reviewed, involved patients with SCD, and provided data about the efficacy and/or effectiveness of hydroxyurea on 1 or more clinically relevant measurable outcomes or described hydroxyurea toxicity in humans with SCD. RCTs published prior to 2007 were included if secondary analyses from the RCTs fulfilled eligibility criteria. We excluded non–English-language articles, review articles, case series, and studies with fewer than 20 patients unless the article was primarily reporting on toxicity. We included toxicity cases if the hydroxyurea dose and duration of treatment could be used to assess for causality. Although the AHRQ review examined barriers to hydroxyurea use, we decided a priori to not address this issue.
We based our data extraction forms on those used in the AHRQ review. Data extraction was done independently and in duplicate by 2 investigators for 4 studies to achieve calibration. These results were compared to achieve consensus, and the data for the remaining studies were extracted independently by a single investigator and reviewed by a second investigator for accuracy. Discrepancies were resolved by discussion or review by a third investigator.
Two investigators independently assessed study quality using the Jadad score for randomized studies10 and the Newcastle-Ottawa scale for nonrandomized studies.11,12 Toxicity reports were assessed using a tool based on 5 questions/criteria developed by the Cochrane Collaboration.13
Detailed data extraction results and assessment of quality are available (supplemental Tables 1-13, available on the Blood Web site). Quality of evidence and recommendations were graded based on Grading of Recommendations Assessment Development and Evaluation tool.14 Disagreements were resolved by discussion or by mediation by a third investigator.
Results
A total of 48 studies were included (Figure 1): 5 RCTs, 11 secondary analyses presenting data from these RCTs, 27 observational studies, and 5 articles that addressed hydroxyurea toxicity (supplemental Tables 1, 4, 7, and 13). All but one RCT15 and its secondary analyses16-20 enrolled patients who had previous sickle cell–related symptoms or complications. Thirty studies enrolled children, 13 enrolled adults, and 5 enrolled both.
Hydroxyurea administration
When specified, all studies initiated orally administered hydroxyurea at doses between 10 and 20 mg/kg per day. Thirteen studies6,21-32 indicated a goal to increase to maximum tolerated dose (limited by mild myelosuppression), 5 studies increased until clinical or laboratory improvement was observed,33-37 and 5 studies used fixed dosing at 10 or 20 mg/kg per day.15,38-41 When stated, all studies used a maximum dose of 30 to 35 mg/kg per day.
Clinical outcomes
In 19 primary studies reporting hematologic data, hydroxyurea therapy was consistently associated with overall increases in HbF.6,15,22,24-26,28,30,32,34-43 Both children and adults with SCA benefited from reductions in the frequency of VOC in 11 studies,6,15,24,30,34-36,38-40,43 ACS in 7 studies,6,15,24,28,34,36,38 and transfusions in 8 studies.6,15,24,36,38-40,43
In children, hydroxyurea was associated with decreased hospitalizations compared with placebo in 2 studies.15,39 In adults and children, decreased rates of hospitalization were reported for patients receiving hydroxyurea compared with prior to hydroxyurea use in 7 studies.28,30,35,36,38,43,44 In 2 observational studies, a reduction in frequency of VOC and in one study in ACS was observed in patients with HbSβ+-thalassemia30,36 (Tables 1 and 2).
RCTs
| Study/authors/location . | Inclusion criteria . | Intervention . | Age mean/median (range) . | N . | Follow-up period . | Outcomes for hydroxyurea treatment vs no hydroxyurea . |
|---|---|---|---|---|---|---|
| MSH*/Charache et al, 1995/North America | SCA; age >18 y; ≥3 crises/y | HU vs placebo; HU: 15 mg/kg per day escalated to MTD | 30 y (18-59) | 299 (HU: 152) | 21 mo (mean); 134/299 with 2 y | 1°: decreased VOC (P < .001); 2°: decreased ACS (P < .001), decreased transfusions (P = .001) |
| BABY HUG/Wang et al, 2011/United States | SCA; 9-18 mo; no requirement for clinical severity | HU vs placebo; HU: fixed at 20 mg/kg per day | 13.6 mo (HU); 13.5 mo (placebo) | 193 (HU: 96) | 24 mo | 1°: no difference in splenic function (P = .21) or renal function (P = .84); 2°: decreased VOC (P = .002), ACS (P = .02), dactylitis (P < .0001) |
| CHAMPS/Wang et al, 2011/United States | HbSC ; age ≥5 y; 1 VOC in previous y | 4 groups: HU+placebo, Mg+placebo, HU+Mg, placebo+placebo; HU: fixed at 20 mg/kg per day | 13.6 y (5-53) | 44 (HU: 22) | 36 patients: 8 wk; 22 patients: 44 wk | Early trial termination due to poor accrual; 1°: no difference in hyperdense red cells; 2°: no difference in frequency of VOC (HU: increased HbF, week 8: P < .05 and week 24: P < .001) |
| Jain et al, 2012/India | SCA; >3 VOC/y; requiring hospitalization or transfusion >3/y | HU vs placebo; HU: fixed at 10 mg/kg per day | 12.7 ± 4.4 y (HU); 11.7 ± 4.1 y (placebo) (5-18) | 60 (HU: 30) | 18 mo | 1°: decreased VOC (P < .001); 2°: decreased transfusions (P < .001); decreased hospitalizations (P < .001) |
| SWiTCH/Ware et al, 2012/United States | SCA | HU/phlebotomy vs txn/chelation (noninferiority trial design); HU: 20 mg/kg per day escalated to MTD | 13y (5-18) | 133 (HU: 67) | 24 mo (mean) | Study closed at first interim analysis; 1°: equivalent liver iron content at interim analysis for both arms; rate of stroke with HU arm within noninferiority margin |
| Study/authors/location . | Inclusion criteria . | Intervention . | Age mean/median (range) . | N . | Follow-up period . | Outcomes for hydroxyurea treatment vs no hydroxyurea . |
|---|---|---|---|---|---|---|
| MSH*/Charache et al, 1995/North America | SCA; age >18 y; ≥3 crises/y | HU vs placebo; HU: 15 mg/kg per day escalated to MTD | 30 y (18-59) | 299 (HU: 152) | 21 mo (mean); 134/299 with 2 y | 1°: decreased VOC (P < .001); 2°: decreased ACS (P < .001), decreased transfusions (P = .001) |
| BABY HUG/Wang et al, 2011/United States | SCA; 9-18 mo; no requirement for clinical severity | HU vs placebo; HU: fixed at 20 mg/kg per day | 13.6 mo (HU); 13.5 mo (placebo) | 193 (HU: 96) | 24 mo | 1°: no difference in splenic function (P = .21) or renal function (P = .84); 2°: decreased VOC (P = .002), ACS (P = .02), dactylitis (P < .0001) |
| CHAMPS/Wang et al, 2011/United States | HbSC ; age ≥5 y; 1 VOC in previous y | 4 groups: HU+placebo, Mg+placebo, HU+Mg, placebo+placebo; HU: fixed at 20 mg/kg per day | 13.6 y (5-53) | 44 (HU: 22) | 36 patients: 8 wk; 22 patients: 44 wk | Early trial termination due to poor accrual; 1°: no difference in hyperdense red cells; 2°: no difference in frequency of VOC (HU: increased HbF, week 8: P < .05 and week 24: P < .001) |
| Jain et al, 2012/India | SCA; >3 VOC/y; requiring hospitalization or transfusion >3/y | HU vs placebo; HU: fixed at 10 mg/kg per day | 12.7 ± 4.4 y (HU); 11.7 ± 4.1 y (placebo) (5-18) | 60 (HU: 30) | 18 mo | 1°: decreased VOC (P < .001); 2°: decreased transfusions (P < .001); decreased hospitalizations (P < .001) |
| SWiTCH/Ware et al, 2012/United States | SCA | HU/phlebotomy vs txn/chelation (noninferiority trial design); HU: 20 mg/kg per day escalated to MTD | 13y (5-18) | 133 (HU: 67) | 24 mo (mean) | Study closed at first interim analysis; 1°: equivalent liver iron content at interim analysis for both arms; rate of stroke with HU arm within noninferiority margin |
1°, primary outcomes; 2°, secondary outcomes; BABY HUG, Pediatric Hydroxyurea in Sickle Cell Anemia; CHAMPS, Hydroxyurea and Magnesium Pidolate to Treat People with Hemoglobin Sickle Cell Disease; HU, hydroxyurea; Mg, magnesium pidolate; MTD, maximum tolerated dose; txn, transfusion.
Secondary analyses of MSH included in the current review.
Observational studies evaluating frequency of acute clinical events in SCD*
| Authors/location . | Patient population/study design . | Genotype/inclusion criteria . | N . | Follow-up period mean/median (range) . | Clinical outcomes HU vs no HU† . | Comments . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| VOC . | ACS . | Transfusion . | Stroke . | Hospital use . | ||||||
| Ali et al, 2011/Jamaica | Children/retrospective | 42 SCA, 1 HbSC; neuroimaging documentation of stroke or dx of clinical stroke | 43 (HU: 10) | HU: 4.6 y; no HU:4.2 y | HU: (2/ 100 p-y); no HU or txn: (29/100 p-y) (HR 9.4, P = .03) | Chronic transfusion program was not available | ||||
| Gilmore et al, 2011/United Kingdom | Children and adults/retrospective | 61 SCA, 1 HbSD; majority-recurrent VOC; VOC with ACS | 62 (HU: 62) | 3 y (1-9) | ↓; P = .02 at 2 y | ↓; P = .0007 at 7 y | ↓; P = .002 at 7 y | Inpatient annual days; ↓; P < .001 at 7 y | Registry data | |
| Italia et al, 2008/India | Children and adults/prospective | 54 HbSS, 23 HbSβthal; Arab-Indian haplotype; 5+ VOC/y, 1 CVA in life, 2+ ACS/life, or (AVN of femur + any of the above | 77 (HU: 77) | 2 y | ↓ | ↓ | ↓ | ↓ | ↓ | High baseline HbF level; reduction in clinical events score stratified according to initial severity (P < .0001) |
| Lobo et al, 2013/Brazil | Children/retrospective | SCD; indications for HU: recurrent VOC; >1 ACS; Hb <6 g/dL, CVA | 1760 (HU: 267 [SCA: 243; HbSB+thal: 10; HbSC: 10; HbSD: 4]) | 7 y (3-17) HU: 2 y (0.1-6.5) | HU:1 no HU: 17 | HU: 0; no HU: 4 | ↓50% P < .001 | ↓ mortality; 1 in HU group; 37 in no-HU group | ||
| ED visits ↓35% P < .001 | ||||||||||
| Nzouakou et al, 2010/France | Adults and adolescents/retrospective | SCA; ≥3 VOC hospitalizations/y; recurrent ACS; recurrent priapism; switch from txn program | 123 (HU: 123) | 4.9 y (0.44-13.5) | ↓; P < .0001 | 15/31 pts stopped txn | ↓ (data on 64 pts); P < .0001 | |||
| Patel et al, 2012/India | Children and adults/prospective | SCA: Asian haplotype; VOC >3 or txn >2 in previous y | 118 (HU: 118) | 2 y | 87.2% with ↓ 50% or more | 19/20 pts txn free | High baseline HbF level | |||
| Rigano et al, 2013/Italy | Adults/retrospective | HbSβ0thal:34; HbSβ+thal:67; 2-3 VOC previous y; hx of ACS | 104 (HU: 104) | 11 y | ↓; P < .0001 | 6.7% overt stroke | ↓; P < .0001 | 30% with new/progressed SCI | ||
| Sharef et al, 2013/Oman | Children/retrospective, prospective | SCD (not specified); >3 VOC hospitalizations/y or 1 episode of ACS | 142 (HU: 142) | 4 y (1.5-10) | ↓ | ↓; P < .001 | ||||
| Singh et al, 2010/India | Adults/prospective | SCA; ≥3VOC hospitalizations/y, ACS, stroke or Hb <7 g/dL | 24 (HU: 24) | 1 y | ↓; P = .008 | ↓; P = .03 | High baseline HbF level | |||
| Steinberg et al, 2010 (MSH)/North America | Adults/prospective | SCA; MSH RCT cohort; analysis by HU use, not original trial assignment | 129/299 of original cohort deceased | Up to 17 y, 7 mo | NS | HU for at least 5 y compared with <5 y had ↓ mortality | ||||
| Voskaridou et al, 2010 (LaSHS)/Greece | Adults/prospective | HbSS: 34, HbSβ0thal: 131, HbSβ+thal: 165; ≥3 VOC/previous y; jaundice; or complications (CVA or ACS in past 5 y) | 330 (HU:131 [SCA:87; SB+thal:44]) | HU: 8 y (0.1-17); no HU: 5 y (0.1-18) | ↓; P < .001 | ↓; P = .016 | ↓; P < .001 | HU: 5; no HU: 10 | ↓; P < .001 | 10 y survival: 86% for HU; 65% for non-HU; P = .001 |
| Authors/location . | Patient population/study design . | Genotype/inclusion criteria . | N . | Follow-up period mean/median (range) . | Clinical outcomes HU vs no HU† . | Comments . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| VOC . | ACS . | Transfusion . | Stroke . | Hospital use . | ||||||
| Ali et al, 2011/Jamaica | Children/retrospective | 42 SCA, 1 HbSC; neuroimaging documentation of stroke or dx of clinical stroke | 43 (HU: 10) | HU: 4.6 y; no HU:4.2 y | HU: (2/ 100 p-y); no HU or txn: (29/100 p-y) (HR 9.4, P = .03) | Chronic transfusion program was not available | ||||
| Gilmore et al, 2011/United Kingdom | Children and adults/retrospective | 61 SCA, 1 HbSD; majority-recurrent VOC; VOC with ACS | 62 (HU: 62) | 3 y (1-9) | ↓; P = .02 at 2 y | ↓; P = .0007 at 7 y | ↓; P = .002 at 7 y | Inpatient annual days; ↓; P < .001 at 7 y | Registry data | |
| Italia et al, 2008/India | Children and adults/prospective | 54 HbSS, 23 HbSβthal; Arab-Indian haplotype; 5+ VOC/y, 1 CVA in life, 2+ ACS/life, or (AVN of femur + any of the above | 77 (HU: 77) | 2 y | ↓ | ↓ | ↓ | ↓ | ↓ | High baseline HbF level; reduction in clinical events score stratified according to initial severity (P < .0001) |
| Lobo et al, 2013/Brazil | Children/retrospective | SCD; indications for HU: recurrent VOC; >1 ACS; Hb <6 g/dL, CVA | 1760 (HU: 267 [SCA: 243; HbSB+thal: 10; HbSC: 10; HbSD: 4]) | 7 y (3-17) HU: 2 y (0.1-6.5) | HU:1 no HU: 17 | HU: 0; no HU: 4 | ↓50% P < .001 | ↓ mortality; 1 in HU group; 37 in no-HU group | ||
| ED visits ↓35% P < .001 | ||||||||||
| Nzouakou et al, 2010/France | Adults and adolescents/retrospective | SCA; ≥3 VOC hospitalizations/y; recurrent ACS; recurrent priapism; switch from txn program | 123 (HU: 123) | 4.9 y (0.44-13.5) | ↓; P < .0001 | 15/31 pts stopped txn | ↓ (data on 64 pts); P < .0001 | |||
| Patel et al, 2012/India | Children and adults/prospective | SCA: Asian haplotype; VOC >3 or txn >2 in previous y | 118 (HU: 118) | 2 y | 87.2% with ↓ 50% or more | 19/20 pts txn free | High baseline HbF level | |||
| Rigano et al, 2013/Italy | Adults/retrospective | HbSβ0thal:34; HbSβ+thal:67; 2-3 VOC previous y; hx of ACS | 104 (HU: 104) | 11 y | ↓; P < .0001 | 6.7% overt stroke | ↓; P < .0001 | 30% with new/progressed SCI | ||
| Sharef et al, 2013/Oman | Children/retrospective, prospective | SCD (not specified); >3 VOC hospitalizations/y or 1 episode of ACS | 142 (HU: 142) | 4 y (1.5-10) | ↓ | ↓; P < .001 | ||||
| Singh et al, 2010/India | Adults/prospective | SCA; ≥3VOC hospitalizations/y, ACS, stroke or Hb <7 g/dL | 24 (HU: 24) | 1 y | ↓; P = .008 | ↓; P = .03 | High baseline HbF level | |||
| Steinberg et al, 2010 (MSH)/North America | Adults/prospective | SCA; MSH RCT cohort; analysis by HU use, not original trial assignment | 129/299 of original cohort deceased | Up to 17 y, 7 mo | NS | HU for at least 5 y compared with <5 y had ↓ mortality | ||||
| Voskaridou et al, 2010 (LaSHS)/Greece | Adults/prospective | HbSS: 34, HbSβ0thal: 131, HbSβ+thal: 165; ≥3 VOC/previous y; jaundice; or complications (CVA or ACS in past 5 y) | 330 (HU:131 [SCA:87; SB+thal:44]) | HU: 8 y (0.1-17); no HU: 5 y (0.1-18) | ↓; P < .001 | ↓; P = .016 | ↓; P < .001 | HU: 5; no HU: 10 | ↓; P < .001 | 10 y survival: 86% for HU; 65% for non-HU; P = .001 |
↓, decreased; AVN, avascular necrosis; CVA, stroke; dx, diagnosis; HbSβ0thal, HbSβ0-thalassemia; HbSβ+thal, HbSβ+-thalassemia; HU, hydroxyurea; hx, history; LaSHS, Laikon Study of Hydroxyurea in Sickle Cell Syndromes; NS, not significant; p-y, patient-years; pts, patients; SCI, silent cerebral infarction; txn, transfusion.
Outcomes evaluated in single studies included cognitive function,62 physical fitness,37 perioperative,27 quality of life,63 retionopathy,23 and tricuspid regurgitant velocity.42
Pre/post comparisons in single group studies.
Evidence for primary stroke prevention was limited to observational data. Three studies found decreased stroke rates in those administered hydroxyurea compared with no treatment (Table 2).28,36,38 Children receiving hydroxyurea had reduced transcranial Doppler (TCD) velocities.20,32 More definitive information was available on the prevention of recurrent stroke. Hydroxyurea was not equivalent to transfusion in the Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) trial based on a composite end point (Table 1).31 In a cohort of children with SCA, hydroxyurea decreased the rate of recurrent stroke compared with no therapy (Table 2).21 In adults with HbSβ0- and HbSβ+-thalassemia, new ischemic neurologic events occurred on hydroxyurea despite effectively reducing VOC and hospitalizations (Table 2).30
Hydroxyurea compared with placebo did not prevent early evidence of renal or splenic dysfunction in toddlers aged 9 to 18 months (Table 1),15 but observational studies in children aged 9 months to 20 years noted significant improvement in splenic uptake, glomerular filtration rate, renal hypertrophy, ability to concentrate urine, microalbuminuria, and retinopathy.16,22,23,26,33
Toxicity
Leukopenia, neutropenia, and thrombocytopenia were the most frequently reported side effects of hydroxyurea.6,15,20,22,28,34,36,38-40,43,45,46 These effects were generally mild and reversible with discontinuation or a decrease in hydroxyurea dose. Other side effects reported include skin and nail changes,6,24,36,39-41,43,45 leg ulcers,36,43,45 and gastrointestinal disturbances6,20,41,46 ; however, the evidence for hydroxyurea being the causative agent of these side effects is not definitive (supplemental Tables 3, 6, and 9).
Concerns about somatic and/or germ line genotoxicity underlie the potential serious late effects of hydroxyurea. Decreased sperm counts, which did not normalize after hydroxyurea cessation, were noted in one study without a comparator group.47 After 17 years of follow-up, 94 pregnancies were reported by female and male subjects enrolled in the Multicenter Study of Hydroxyurea in Sickle Cell Anemia (MSH) regardless of their HU exposure.48 Of female subjects with known HU exposure at conception or during gestation and by male subjects with known exposure at the time of conception, 16 pregnancy outcomes were reported with 8 live births (6 term and 2 premature deliveries), 5 elective abortions, and 3 spontaneous abortions. Of the live births, no birth defects were described consistent with findings in 3 other studies reporting pregnancy outcomes24,36,47 (supplemental Table 9).
Five malignancies were reported in 951 patients taking hydroxyurea (0.5%) and 1 malignancy among 1736 patients not taking hydroxyurea (0.06%).24,28,34,36,43,49 Three case reports were published after 2007 describing a hematologic malignancy presenting 4 to 15 years after starting hydroxyurea in patients with SCD.50-52 Ex vivo studies of mononuclear cells taken from patients on hydroxyurea did not demonstrate increased genomic instability that might contribute to teratogenesis or leukemogenesis.18,29
Mortality
Hydroxyurea treatment was associated with decreased mortality in symptomatic patients with SCA compared with patients receiving shorter-term hydroxyurea or no hydroxyurea (Table 2).49 Decreased mortality was also seen in 2 other cohort studies among pediatric28 and adult36 patients with SCA receiving hydroxyurea, which was not statistically significant for patients with HbSβ+-thalassemia in adults.
Recommendations
In adults with SCA who have ≥3 moderate to severe pain episodes in a 12-month period, we recommend hydroxyurea therapy (grade 1A).
In adults with SCA who have a history of ACS or symptomatic anemia, we recommend hydroxyurea therapy (grade 1B).
In children with SCA who have ≥3 moderate to severe pain episodes in a 12-month period, we recommend hydroxyurea therapy (grade 1B).
In children with SCA who have a history of ACS or symptomatic anemia, we recommend hydroxyurea therapy (grade 1B).
For infants and children age 9 months or older with SCA who are asymptomatic or have infrequent pain episodes, we recommend hydroxyurea therapy (grade 1B). We acknowledge the improvements in outcome are based on secondary outcomes from a RCT, which may or may not hold value for an individual patient.
In patients with SCA who have a history of stroke and a contraindication to chronic transfusions, we suggest hydroxyurea therapy as compared with no therapy (grade 2B).
In adults with HbSβ+-thalassemia with ≥3 moderate to severe pain episodes in a 12-month period or a history of ACS, we suggest hydroxyurea therapy (grade 2C).
In patients with HbSC and children with HbSβ+-thalassemia, there is insufficient evidence to provide recommendations on hydroxyurea therapy.
Discussion
A systematic review of published literature from 2007 to 2013 was performed to update the evidence review sponsored by the AHRQ.8 The current literature provides a substantive increase in data on children and long-term outcomes for adults with SCD receiving hydroxyurea. Our recommendations are based on the evidence derived from both this current and the AHRQ systematic reviews.
To date, only 5 RCTs have addressed the efficacy of hydroxyurea in patients with SCA.6,15,31,39,53 Thus, evidence gathered using other study designs substantially informs our recommendations. With the exception of the SWiTCH trial, these other RCTs described a benefit for patients receiving hydroxyurea compared with the standard of care.31 The MSH study reported reduced frequency of VOC, ACS, and transfusions.6 A Belgian RCT with a crossover design found fewer VOC, hospitalizations, and hospital days in children receiving hydroxyurea.53 Although BABY HUG did not demonstrate prevention or improvement of splenic or renal dysfunction with hydroxyurea, differences were seen in secondary end points with significant decreases in pain and dactylitis as well as moderate decreases in ACS, hospitalization rates, and transfusion.15 A small RCT from India reported a significant decrease in the number of VOC, transfusion requirements, and hospitalizations with hydroxyurea compared with placebo despite high baseline HbF levels and low-dose therapy.39 Findings from nonrandomized studies are consistent with those from RCTs in SCA, confirming decreased frequency of VOC, ACS, transfusion, and hospitalizations as well as overall safety of hydroxyurea in both children and adults. Furthermore, the long-term observational studies support the sustainability of effectiveness. Mortality is difficult to assess in the RCTs due to the limited follow-up period. However, results from 3 prospective observational studies with substantial sample size for trials in SCA (2 in adults, 1 in children) indicate a survival advantage for patients receiving hydroxyurea.28,36,49
The strength of the evidence and serious consequences of frequent VOC or ACS provide the basis for our strong recommendations for hydroxyurea use in treating adults and children with SCA for these indications. There are no phase 3 RCTs enrolling patients with genotypes other than HbSS and HbSβ0-thalassemia. Evidence from cohort studies of patients from Greece and Italy with HbSβ+-thalassemia extend the findings for SCA to this population; however, the recommendation to use hydroxyurea is weak based on a relatively small sample size compared with data on SCA.30,36 Furthermore, in the Greek cohort, the majority of patients had the IVSI-110 mutation, which is associated with more severe disease than those of African ancestry with HbSβ+-thalassemia.36 The recommendation to use hydroxyurea treatment in asymptomatic children with SCA is based on secondary outcomes of BABY HUG and safety data from previously reported and currently cited studies.8,15 In those children and adults who have experienced prior stroke or children at risk of initial stroke based on elevated TCD velocity, chronic blood transfusion is the standard of care to prevent recurrence or initial stroke.54 Findings from the SWiTCH trial add support to this recommendation. The upcoming TCD with Transfusions Changing to Hydroxyurea (TWiTCH) study is a National Heart Lung and Blood Institute (NHLBI)-sponsored, phase 3, multicenter trial that will compare monthly transfusions, the current established treatment, to daily hydroxyurea to reduce the risk of primary stroke in children with SCA and abnormal TCD velocities.55 However, in circumstances where transfusion is not available or is contraindicated, lower-quality evidence suggests use of hydroxyurea rather than no therapy.21,25 For patients with HbSC, the paucity of clinical data despite encouraging laboratory findings with hydroxyurea, does not allow us to make a recommendation for or against treatment in any clinical scenario.41
Two sets of clinical guidelines published in 2014 address the use of hydroxyurea in SCD.56,57 Our recommendations for hydroxyurea therapy are generally aligned with those presented in the NHLBI-sponsored Evidence-Based Management of Sickle Cell Disease Expert Panel Report.56 The American Thoracic Society guidelines on the management of pulmonary hypertension in SCD recommend hydroxyurea for patients identified as being at increased risk for mortality.57 The risk assessment includes elevated tricuspid regurgitation jet velocity, elevated brain natriuretic peptide, or documented pulmonary hypertension. We found no direct evidence for the efficacy of hydroxyurea for these particular indications.
Hydroxyurea is currently the only US Food and Drug Administration–approved medication to modify the disease course in SCD. Studies reviewed herein provide increasing support of efficacy, effectiveness, and safety in both children and adults, yet indicate serious underuse for indications with the strongest evidence.8,9,58,59 Although data on toxicity associated with hydroxyurea therapy in SCD from our review are derived from case reports or series and clinical studies where toxicities were not systematically assessed, findings are consistent with the assessment in the AHRQ review.8 To highlight an example, the consideration of malignancy associated with hydroxyurea has been identified as a potential barrier to its use.60,61 The identification of 3 additional case reports of hematologic malignancies given the substantial increase in patient-years of exposure based on inclusion of more recent studies and ex vivo investigations does not provide adequate evidence of this increased risk. The decision to start hydroxyurea can be effectively based on patient and family preferences after discussing benefits and risks in the context of assuring a clear understanding of the significant burden of disease and early mortality associated with SCD.
Cases revisited
In cases 1 and 2 we recommend and in case 3 we suggest hydroxyurea therapy.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank George Buchanan, Joylene John-Sowah, Craig Kitchens, Abdullah Kutlar, Merry Jennifer Markham, Anita Rajasekhar, Lisa Richardson, Barbara Yawn, and Marc Zumberg for reviewing the manuscript and providing recommendations. R.L. was a panel member on the NHLBI-sponsored Evidence-Based Management of Sickle Cell Disease Expert Panel Report.
Authorship
Contribution: T.E.W. reviewed the articles, developed outcome tables, performed data extraction and quality assessments, analyzed the data, and wrote the manuscript; A.M.B. performed the article search, reviewed the articles, developed outcome tables, performed data extraction and quality assessments, analyzed the data, and wrote the manuscript; W.L. designed the study, performed the article search, developed outcome tables, analyzed the data, and wrote the manuscript; and R.L. conceived the study, developed outcome tables, performed data extraction and quality assessments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Lottenberg, Division of Hematology/Oncology, Department of Medicine, University of Florida, Box 100278, Gainesville, FL 32610-0278; e-mail: lottenr@medicine.ufl.edu.