Key Points
Sialylated O-glycans protects PDPN from proteolytic degradation.
Sialylated core 1 O-glycans of PDPN on lymphatic endothelial cells (LECs) are required for interacting with platelets.
Abstract
O-glycosylation of podoplanin (PDPN) on lymphatic endothelial cells is critical for the separation of blood and lymphatic systems by interacting with platelet C-type lectin-like receptor 2 during development. However, how O-glycosylation controls endothelial PDPN function and expression remains unclear. In this study, we report that core 1 O-glycan–deficient or desialylated PDPN was highly susceptible to proteolytic degradation by various proteases, including metalloproteinases (MMP)-2/9. We found that the lymph contained activated MMP-2/9 and incubation of the lymph reduced surface levels of PDPN on core 1 O-glycan–deficient endothelial cells, but not on wild-type ECs. The lymph from mice with sepsis induced by cecal ligation and puncture, which contained bacteria-derived sialidase, reduced PDPN levels on wild-type ECs. The MMP inhibitor, GM6001, rescued these reductions. Additionally, GM6001 treatment rescued the reduction of PDPN level on lymphatic endothelial cells in mice lacking endothelial core 1 O-glycan or cecal ligation and puncture-treated mice. Furthermore, core 1 O-glycan–deficient or desialylated PDPN impaired platelet interaction under physiological flow. These data indicate that sialylated O-glycans of PDPN are essential for platelet adhesion and prevent PDPN from proteolytic degradation primarily mediated by MMPs in the lymph.
Introduction
Podoplanin (PDPN), a type 1 transmembrane mucin-type O-glycoprotein, was initially discovered as a platelet aggregation-inducing glycoprotein expressed on tumor cells.1 Subsequent studies have shown that it is expressed on several cell types, including lymphatic endothelial cells (LECs).2-5 PDPN on LECs is critical for the initiation and maintenance of an independent lymphatic vascular system.
PDPN consists of 172 amino acids in mice and 163 amino acids in humans. It has an extracellular domain, a transmembrane domain, and a short cytoplasmic domain. A striking feature of the extracellular domain of PDPN is a high content of serine and threonine residues that can potentially be attached by mucin-type O-glycans (also known as O-N-acetylgalactosamine [GalNAc] glycans or simply O-glycans for this study).5-7 O-glycosylation is a common form of posttranslational modification of membrane and secreted proteins.8-10 It occurs in the Golgi apparatus via sequential reactions catalyzed by specific glycosyltransferases. The core of all mucin-type O-glycans is serine/threonine-linked GalNAc, also known as the Tn antigen, which is normally not exposed due to further modification in forming distinct subtypes of O-glycans. Among them, core 1 O-glycans are a predominant form. Core 1 O-glycans are synthesized by adding galactose in β3 linkage to Tn antigen, which is catalyzed solely by the T-synthase (core 1 synthase, C1galt1).8-11 Core 1 structure can be further branched to form extended core 1 or core 2 structures, or can be modified by adding sialic acids. These glycans are known as core 1-derived O-glycans.10,11
The molecular weight of the core PDPN polypeptide is about 17 kDa; however, PDPN isolated from different cell types has an apparent molecular weight ranging from 37 kDa to 41 kDa, suggesting extensive, heterogeneous O-glycosylation. In our previous study, mice lacking endothelial core 1 O-glycans display impaired PDPN expression that is required for the development and maintenance of an independent lymphatic vascular system.8,12,13 PDPN binding to the platelet C-type lectin-like receptor 2 (CLEC-2) induces platelet aggregation that seals initial blood-lymphatic vascular connections during embryonic development.12 Sialyl core 1 O-glycans on PDPN are required for interacting with CLEC-2 using Chinese hamster ovary cells (CHO) or synthetic glycopeptides.6,7,14 However, CHO cells lack core 2 and extended core 1 O-glycans.15 Therefore, whether PDPN on endothelial cells (ECs) requires similar O-glycosylation to interact with CLEC-2 is unclear. Additionally, how O-glycosylation regulates function and expression of PDPN on LECs remains to be addressed.
In this study, we show that core 1 O-glycan–deficient or desialylated PDPN on LECs is highly susceptible to proteolytic degradation, suggesting the importance of O-glycosylation for controlling stability on EC surfaces. Notably, matrix metalloproteinases (MMPs) in the lymph are essential for the proteolytic degradation of core 1 O-glycan–deficient or desialylated PDPN. Furthermore, core 1 O-glycan–deficient or desialylated PDPN on LECs exhibits impaired interactions with platelets under flow. Our results provide strong evidence that sialylated O-glycans control the expression and function of endothelial PDPN.
Materials and methods
Mice and cells
Mice lacking EC and hematopoietic core (EHC) 1 O-glycans (C1galt1−/− or C1galt1f/f; Tie2Cre) and platelet-specific Clec2−/− mice were described previously.8,13 Mice with doxycycline-inducible global deficiency of core 1 O-glycans (inducible C1galt1−/−) were generated by crossing C1galt1f/f mice with Rosa26-rtTA, tetO-Cre Tg mice (The Jackson Laboratory). Primary LECs were isolated from different tissues as described in “Results.” Some of the LECs were used for culture. Stable PDPN-eGFP–expressed ECs are established by transfection of immortalized wild-type (WT) or C1galt1−/− ECs8 with DNA construct of murine PDPN tagged with a C-terminal eGFP (PDPN-eGFP). CHO cells were cotransfected with complementary DNA constructs encoding PDPN-eGFP and glycosyltransferases. All mouse experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Platelet adhesion under flow
WT or Clec2−/− platelet adhesion on ECs or CHO cells expressing PDPN-eGFP were performed under flow.16
Lymph or protease treatment
PDPN-eGFP transfected WT or C1galt1−/− ECs were incubated with 200 μL of mouse lymph or buffer containing proteases.
Intraperitoneal (IP) injection of MMP inhibitor
IP injections of GM6001 were performed. Primary LECs isolated from mesentery and cryosections of ileum were used for staining to examine Tn, Lyve-1, and PDPN expression.
Cecal ligation and puncture (CLP) sepsis model
CLP was performed with or without IP injection of sialidase inhibitor or GM6001. The lymph and mesentery were collected for EC treatment and flow cytometry.
More details of materials and methods used are described in the supplemental Methods, available on the Blood Web site.
Statistics
The unpaired Student t test was used to determine P values as indicated in the figures.
Results
Lack of core 1 O-glycosylation reduces PDPN on LECs in vivo
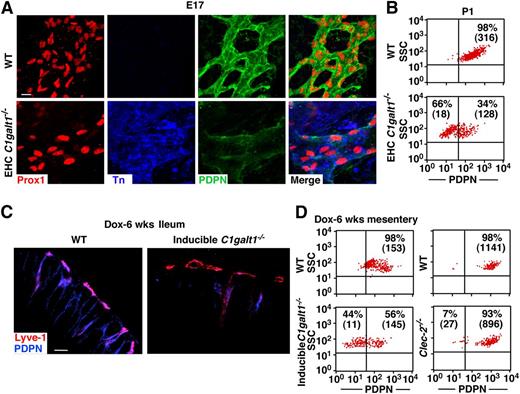
We report here that mice lacking core 1 O-glycans (EHC C1galt1−/−) exhibited reduced levels of PDPN in lymphatic microvessels.8 To determine whether the reduction of PDPN occurred on larger collecting lymphatic vessels, we performed immunostaining on lymphatic vessels in embryonic day (E17) mesentery. We first probed the Prox1-positive LECs of the collecting lymphatic vessels of EHC C1galt1−/− mesenteries with mAb to Tn antigen (Figure 1A).8,9 Anti-Tn mAb stained C1galt1−/− but not WT LECs, indicating efficient excision of the C1galt1 gene. Desialylation did not appreciably affect the intensity of anti-Tn staining (data not shown), indicating that most exposed Tn is not sialylated. Immunofluorescent images revealed that the collecting lymphatic vessels of EHC C1galt1−/− mesenteries had reduced PDPN levels relative to WT collecting lymphatic vessels. Using primary LECs, which were defined as CD31+/Lyve-1+ cells (supplemental Figure 1A), flow cytometric analyses showed that the surface level of PDPN on mesenteric LECs from EHC C1galt1−/− mice was reduced compared with that from WT mice (Figure 1B). Reduced surface levels of PDPN were also found on isolated primary LECs from collecting lymphatic vessels in 3-week-old and 8-week-old EHC C1galt1−/− mesentery (supplemental Figure 1B), on isolated primary LECs from P1 (supplemental Figure 1C), or in cryosections of lymphatic microvessels of 8-week-old EHC C1galt1−/− small intestine (supplemental Figure 1D). These results indicate that loss of core 1 O-glycans causes defective expression of PDPN in different types of C1galt1−/− lymphatic vessels.
Lack of core 1 O-glycans causes reduced PDPN levels on LECs. (A) Whole mount images of WT or EHC C1galt1−/− mouse mesentery at E17 with antibodies against Prox-1, Tn, and PDPN. Scale bar, 10 μm. (B) Flow cytometric analyses of cell surface PDPN levels on freshly isolated mesenteric LECs from WT or EHC C1galt1−/− mice at P1. Dot plots are shown after gating on CD31+/Lyve-1+ LEC cells. Percentage and mean fluorescence intensity (MFI) (in parenthesis) are indicated for PDPN in each quadrant. (C, left) C1galt1f/f;tetO-Cre+ mice and littermate WT mice were fed a doxycycline diet for 6 weeks, starting at 6-weeks old. Confocal images of ileal cryosections from doxycycline-inducible or littermate control mice with antibodies against PDPN and Lyve-1 are shown. Scale bar, 20 μm. (C, right) Flow cytometric analyses of PDPN on CD31+/Lyve-1+ isolated mesenteric LECs from doxycycline-inducible or littermate control mice, and (D) CD31+/Lyve-1+ isolated mesenteric LECs from 12-week-old WT or Clec2−/− mice. The images and data are representative of at least 3 experiments. E17, embryonic day 17; P1, postnatal day. SSC, side scatter.
Lack of core 1 O-glycans causes reduced PDPN levels on LECs. (A) Whole mount images of WT or EHC C1galt1−/− mouse mesentery at E17 with antibodies against Prox-1, Tn, and PDPN. Scale bar, 10 μm. (B) Flow cytometric analyses of cell surface PDPN levels on freshly isolated mesenteric LECs from WT or EHC C1galt1−/− mice at P1. Dot plots are shown after gating on CD31+/Lyve-1+ LEC cells. Percentage and mean fluorescence intensity (MFI) (in parenthesis) are indicated for PDPN in each quadrant. (C, left) C1galt1f/f;tetO-Cre+ mice and littermate WT mice were fed a doxycycline diet for 6 weeks, starting at 6-weeks old. Confocal images of ileal cryosections from doxycycline-inducible or littermate control mice with antibodies against PDPN and Lyve-1 are shown. Scale bar, 20 μm. (C, right) Flow cytometric analyses of PDPN on CD31+/Lyve-1+ isolated mesenteric LECs from doxycycline-inducible or littermate control mice, and (D) CD31+/Lyve-1+ isolated mesenteric LECs from 12-week-old WT or Clec2−/− mice. The images and data are representative of at least 3 experiments. E17, embryonic day 17; P1, postnatal day. SSC, side scatter.
Loss of core 1 O-glycans occurs during embryonic development in EHC C1galt1−/− mice. To determine whether postnatal loss of core 1 O-glycosylation impairs PDPN expression, we used inducible C1galt1−/− mice. Six weeks after a doxycycline diet, Tn antigen was detected in the intestine of inducible C1galt1−/− mice but not in WT littermates (supplemental Figure 1E). Lyve-1–positive submucosal lymphatic vessels of the WT intestine expressed a high level of PDPN (Figure 1C, left). In contrast, the level of PDPN was diminished in those of the inducible C1galt1−/− intestine. Flow cytometric analyses showed reduced PDPN levels on primary LECs from the inducible C1galt1−/− mesentery compared with that from WT littermates (Figure 1C, right). These data indicate that reduced PDPN levels in lymphatic vessels of EHC C1galt1−/− mice occur during both developmental and postnatal stages.
EHC C1galt1−/− mice exhibit disorganized and blood-filled lymphatic vessels.8 To determine whether these lymphatic vascular abnormalities contribute to defective PDPN expression, we examined PDPN levels on LECs isolated from mice lacking CLEC-2 (Clec2−/−), which exhibit the same lymphatic vascular abnormalities.12,13 PDPN expression on LECs from Clec2−/− mice was equivalent to that from WT mice, indicating that lymphatic abnormalities do not cause the reduced PDPN expression (Figure 1D). WT and C1galt1−/− LECs expressed a similar amount of PDPN messenger RNA, suggesting that lack of core 1 O-glycans does not affect the transcription (supplemental Figure 2). Collectively, these data indicate that lack of core 1 O-glycans causes a reduction of PDPN in C1galt1−/− lymphatic vessels.
Lack of core 1 O-glycosylation does not affect intracellular trafficking or protein turnover of PDPN
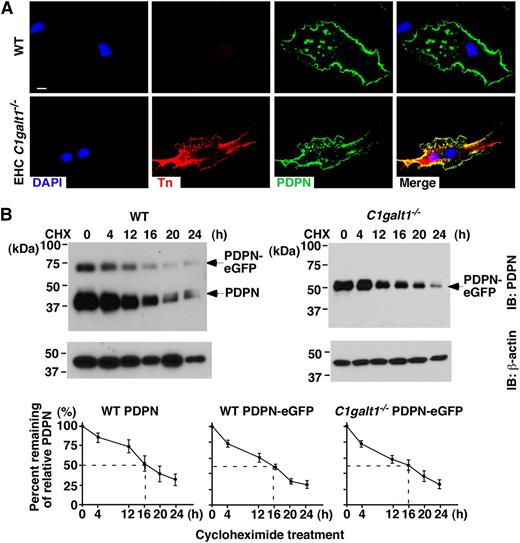
O-glycan deficiency may result in defective synthesis, impaired intracellular trafficking, or increased degradation of glycoproteins.8,17-24 To determine how the lack of core 1 O-glycan resulted in reduced expression of PDPN, we isolated primary skin ECs enriched in LECs from WT or EHC C1galt1−/− mice,8 and then cultured the isolated cells for 5 days to increase cell numbers for analysis (referring to cultured primary LECs). Most of these cells were CD31 and Lyve-1 positive, consistent with their LEC phenotype (supplemental Figure 3). Anti-Tn antibody stained C1galt1−/− but not WT cultured primary LECs (Figure 2A). Immunofluorescent staining of permeabilized cells revealed similar levels of PDPN inside the WT and C1galt1−/− cultured primary LECs, indicating that the lack of O-glycans does not affect the intracellular localization of PDPN.
Lack of core 1 O-glycans does not affect intracellular trafficking or turnover of PDPN in vitro. (A) Confocal images of cultured primary LECs from WT or EHC C1galt1−/− mouse skin, with antibodies against PDPN and Tn antigen. DAPI indicates cell nuclei. Scale bar, 5 μm. (B) WT or C1galt1−/− ECs transfected with PDPN-eGFP were treated with 100 μg/ml CHX for the indicated time. A total of 20 μg of lysates was run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-PDPN (8.1.1) and anti-actin antibodies. The relative PDPN intensity is calculated by normalization of PDPN band intensity with actin band intensity from the same lysate. Marked line charts are presented as remainder of relative PDPN intensity at the indicated time points against that at 0 hours of treatment. The data represent the mean ± standard deviation (SD) from 4 experiments. CHX, cycloheximide.
Lack of core 1 O-glycans does not affect intracellular trafficking or turnover of PDPN in vitro. (A) Confocal images of cultured primary LECs from WT or EHC C1galt1−/− mouse skin, with antibodies against PDPN and Tn antigen. DAPI indicates cell nuclei. Scale bar, 5 μm. (B) WT or C1galt1−/− ECs transfected with PDPN-eGFP were treated with 100 μg/ml CHX for the indicated time. A total of 20 μg of lysates was run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-PDPN (8.1.1) and anti-actin antibodies. The relative PDPN intensity is calculated by normalization of PDPN band intensity with actin band intensity from the same lysate. Marked line charts are presented as remainder of relative PDPN intensity at the indicated time points against that at 0 hours of treatment. The data represent the mean ± standard deviation (SD) from 4 experiments. CHX, cycloheximide.
Next, we compared the stability of PDPN using PDPN-eGFP transfected WT or C1galt1−/− EC lines.8 Immortalized WT ECs expressed endogenous PDPN, but C1galt1−/− ECs had lost their endogenous PDPN at the messenger RNA level (supplemental Figure 4A). After transfection with PDPN-eGFP, PDPN expressed on the WT and C1galt1−/− EC surfaces was detected similarly by Syrian hamster anti-mouse PDPN monoclonal antibody (mAb, clone 8.1.1), indicating that the antibody binds to PDPN in an O-glycosylation–independent manner (supplemental Figure 4B). Both ECs expressed endothelial (CD31) and lymphatic (Lyve-1) markers. Tn antigen was expressed in C1galt1−/− ECs, indicating the loss of core 1 O-glycans (supplemental Figure 4C). The WT ECs expressed endogenous PDPN (38 kDa) and PDPN-eGFP (64 kDa), whereas the C1galt1−/− ECs only expressed an underglycosylated form of the transfected PDPN-eGFP (52 kDa) due to lack of the core 1 O-glycan (Figure 2B). PDPN protein synthesis was inhibited by cycloheximide, and the decay of PDPN over time was determined by immunoblot analysis. WT and core 1 O-glycan–deficient PDPN-eGFP, as well as endogenous WT PDPN, were similarly reduced in level by 50% at ∼16 hours. The internal control β-actin remained stable for about 20 hours, consistent with published results.25 Taken together, these results support that lack of core 1 O-glycosylation does not affect intracellular synthesis, trafficking, or protein turnover of PDPN.
Core 1 O-glycans protect PDPN from proteolytic degradation
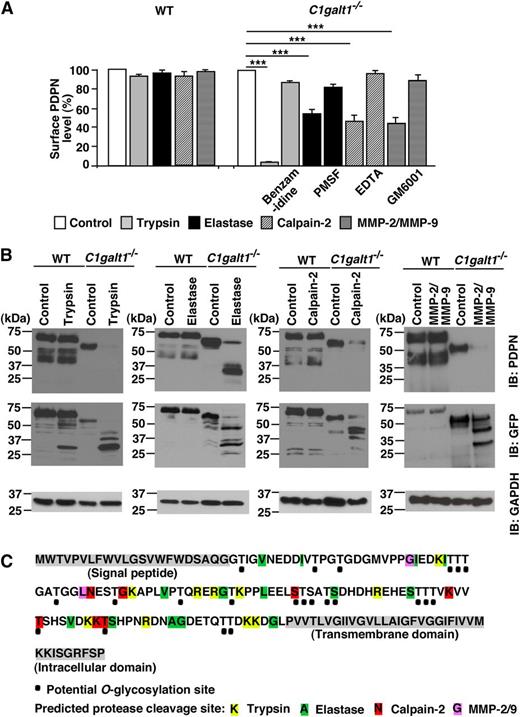
To test whether core 1 O-glycan–deficient PDPN is sensitive to proteolytic degradation, we first treated the PDPN-transfected ECs described above with serine protease trypsin. Flow cytometry demonstrated that trypsin treatment significantly reduced the PDPN level on the surfaces of C1galt1−/− but not WT ECs. This reduction was rescued by trypsin-like serine protease inhibitor benzamidine, illustrating its specificity (Figure 3A). The WT or C1galt1−/− ECs with buffer control or trypsin treatment were also lysed for immunoblot analyses (Figure 3B). Control WT EC lysates exhibited two major bands when probed with anti-PDPN (8.1.1). Although 8.1.1 detected no obvious degraded PDPN, anti-GFP antibody recognized a 30-kDa band from lysates of trypsin-treated WT ECs, suggesting that trypsin generated a minor cleaved product of WT PDPN under the conditions tested in this study. Control core 1 O-glycan–deficient PDPN from the C1galt1−/− ECs was blotted around 52 kDa with 8.1.1, whereas 8.1.1 did not detect PDPN from the trypsin-treated C1galt1−/− ECs. These results suggest that trypsin degraded the core 1 O-glycan–deficient PDPN and eliminated epitopes recognized by the 8.1.1 antibody. Consistent with this, anti-GFP antibody detected several fragments of core 1 O-glycan–deficient PDPN after trypsin treatment. To confirm these results, we used acomplementary cell surface biotinylation method (supplemental Figure 5A). Surface-biotinylated nontransfected WT ECs or transfected stable C1galt1−/− ECs were lysed, and then PDPN in cell lysates was immunoprecipitated with anti-PDPN 8.1.1-conjugated agarose beads. The beads were treated with trypsin or buffer control. Immunoblots using the eluted proteins demonstrated that the control nontransfected WT ECs expressed only endogenous 38 kDa PDPN, whereas the control transfected C1galt1−/− ECs expressed 52 kDa PDPN-eGFP. Trypsin treatment resulted in a partial degradation of WT PDPN, whereas it degraded all core 1 O-glycan–deficient PDPN, as no intact PDPN was detected after treatment. Further analyses indicated that core 1 O-glycan–deficient PDPN was also highly susceptible to degradation by another serine protease, elastase (Figure 3A-B), but not by thrombin, plasmin, or cathepsin-G (supplemental Figure 5B).
Core 1 O-glycans protect PDPN from proteolytic degradation. (A) Stable PDPN-eGFP transfected WT or C1galt1−/− ECs were incubated in buffer (control), 0.05% trypsin (with or without 5 mM benzamidine), 100 μg/ml elastase (with or without 2 mM PMSF), 10 μg/ml calpain-2 (with 2 mM Ca++, with or without 5 mM EDTA), or 2 μg/ml activated MMP-2/MMP-9 (with or without 100 μM GM6001), and then stained with anti-PDPN antibody for flow cytometry. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 to 5 experiments. ***P < .001. (B) Stable WT or C1galt1−/− ECs were treated with or without trypsin, elastase, calpain-2, or activated MMP-2/MMP-9, and then lysed. All lysates were analyzed by immunoblot using anti-PDPN mAb (8.1.1) or rabbit anti-GFP Ab. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. (C) Potential O-glycosylation sites (black dots) and predicted cleavage sites of PDPN by trypsin (yellow), elastase (green), calpain-2 (red), or MMP-2/MMP-9 (purple) in the extracellular domain of PDPN.
Core 1 O-glycans protect PDPN from proteolytic degradation. (A) Stable PDPN-eGFP transfected WT or C1galt1−/− ECs were incubated in buffer (control), 0.05% trypsin (with or without 5 mM benzamidine), 100 μg/ml elastase (with or without 2 mM PMSF), 10 μg/ml calpain-2 (with 2 mM Ca++, with or without 5 mM EDTA), or 2 μg/ml activated MMP-2/MMP-9 (with or without 100 μM GM6001), and then stained with anti-PDPN antibody for flow cytometry. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 to 5 experiments. ***P < .001. (B) Stable WT or C1galt1−/− ECs were treated with or without trypsin, elastase, calpain-2, or activated MMP-2/MMP-9, and then lysed. All lysates were analyzed by immunoblot using anti-PDPN mAb (8.1.1) or rabbit anti-GFP Ab. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. (C) Potential O-glycosylation sites (black dots) and predicted cleavage sites of PDPN by trypsin (yellow), elastase (green), calpain-2 (red), or MMP-2/MMP-9 (purple) in the extracellular domain of PDPN.
To determine whether core 1 O-glycan–deficient PDPN is susceptible to other types of proteases, we examined cysteine protease calpain-2 and matrix MMP-2/9. Our results indicated that calpain-2 and MMP-2/9 reduced PDPN levels on the C1galt1−/− but not the WT ECs (Figure 3A). EDTA treatment rescued these reductions because these enzymes are divalent cation-dependent. Additionally, the MMP inhibitor GM6001 rescued PDPN expression following MMP-2/MMP-9 treatment. These experiments demonstrated the specificity of these proteolytic degradations. Further immunoblot analyses with anti-PDPN and anti-GFP indicated that MMP-2/9 degraded core 1 O-glycan–deficient PDPN, whereas WT PDPN remained largely intact (Figure 3B). Similar results were seen with calpain-2 treatment. These results are consistent with analyses showing that the extracellular domain of PDPN has predicted cleavage sites for trypsin, elastase, calpain-2, and MMP-2/9, but not for thrombin or cathepsin-G (Figure 3C). Interestingly, most of the predicted protease cleavage sites were located in close proximity to the potential O-glycosylation sites of PDPN. These data suggest that a key function of O-glycosylation is to protect glycoproteins from proteolytic degradation.
MMPs are responsible for the degradation of core 1 O-glycan–deficient PDPN
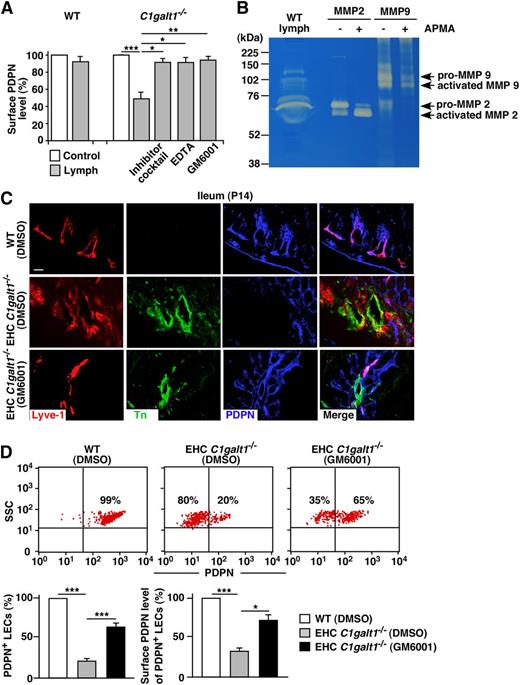
To determine the source and identities of the proteases responsible for degrading core 1 O-glycan–deficient PDPN in vivo, we tested lymph isolated from WT mice because PDPN on LEC surfaces was present in the fluid. Lymph significantly reduced the PDPN level on the C1galt1−/− but not on the WT ECs (Figure 4A), which was prevented in the presence of a protease inhibitor cocktail, demonstrating that the reduction of PDPN by lymph is protease-dependent. Importantly, while GM6001 or EDTA completely rescued the reduction of PDPN on the lymph-treated C1galt1−/− ECs, benzamidine or phenylmethylsulfonyl fluoride (PMSF) rescued PDPN to a lesser degree (supplemental Figure 6). GM6001 did not block the activity of trypsin, elastase, or calpain-2 (supplemental Figure 7), thereby supporting its specificity. MMP-2/9, mainly activated MMP-2, was detected in WT lymph by gelatin zymography (Figure 4B), but not in the conditioned medium of cultured ECs (supplemental Figure 8). Collectively, these data demonstrate that matrix MMPs in the lymph not derived from PDPN-expressing ECs, contribute to the degradation of core 1 O-glycan–deficient PDPN on C1galt1−/− LECs.
MMPs in the lymph cleave core 1 O-glycan–deficient PDPN in vitro and in vivo. (A) Stable PDPN-eGFP WT or C1galt1−/− ECs were incubated in culture medium (as a control), lymph only, lymph with a 1:100 diluted protease inhibitor cocktail, with 5 mM EDTA, or with 100 mM GM6001, and then labeled with anti-PDPN antibody for flow cytometry. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. Data represent the mean ± SD from 3 experiments. (B) Lymph from C57BL/6J mice was analyzed for MMP-2 and MMP-9 by gelatin zymography. Purified mouse pro–MMP-2 and recombinant mouse pro–MMP-9 were activated by APMA and used as positive controls. Samples were resolved in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels containing 1 mg/mL gelatin, then visualized by staining with Coomassie Brilliant Blue G-250. (C) Confocal images of intestinal cryosections from vehicle-treated WT, vehicle-treated EHC C1galt1−/−, or GM6001-treated EHC C1galt1−/− mice stained with antibodies against Lyve-1, Tn, and PDPN. Scale bar, 20 μm. (D, top) Flow cytometric analyses of cell surface PDPN on freshly isolated mesenteric LECs from vehicle-treated WT, vehicle-treated EHC C1galt1−/−, or GM6001-treated EHC C1galt1−/−mice. PDPN expression was analyzed on the CD31+/Lyve-1+ LEC population. (D, bottom) Quantification of the percentages of PDPN+ LEC numbers (left) and percentages of MFIs of PDPN on LECs (right) are shown. MFIs of PDPN on LECs from vehicle-treated EHC C1galt1−/− or GM6001-treated EHC C1galt1−/− mice were normalized to the MFI of PDPN on LECs from vehicle-treated WT mice. Data represent the mean ± SD from 5 experiments. *P < .05; **P < .01; ***P < .001.
MMPs in the lymph cleave core 1 O-glycan–deficient PDPN in vitro and in vivo. (A) Stable PDPN-eGFP WT or C1galt1−/− ECs were incubated in culture medium (as a control), lymph only, lymph with a 1:100 diluted protease inhibitor cocktail, with 5 mM EDTA, or with 100 mM GM6001, and then labeled with anti-PDPN antibody for flow cytometry. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. Data represent the mean ± SD from 3 experiments. (B) Lymph from C57BL/6J mice was analyzed for MMP-2 and MMP-9 by gelatin zymography. Purified mouse pro–MMP-2 and recombinant mouse pro–MMP-9 were activated by APMA and used as positive controls. Samples were resolved in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels containing 1 mg/mL gelatin, then visualized by staining with Coomassie Brilliant Blue G-250. (C) Confocal images of intestinal cryosections from vehicle-treated WT, vehicle-treated EHC C1galt1−/−, or GM6001-treated EHC C1galt1−/− mice stained with antibodies against Lyve-1, Tn, and PDPN. Scale bar, 20 μm. (D, top) Flow cytometric analyses of cell surface PDPN on freshly isolated mesenteric LECs from vehicle-treated WT, vehicle-treated EHC C1galt1−/−, or GM6001-treated EHC C1galt1−/−mice. PDPN expression was analyzed on the CD31+/Lyve-1+ LEC population. (D, bottom) Quantification of the percentages of PDPN+ LEC numbers (left) and percentages of MFIs of PDPN on LECs (right) are shown. MFIs of PDPN on LECs from vehicle-treated EHC C1galt1−/− or GM6001-treated EHC C1galt1−/− mice were normalized to the MFI of PDPN on LECs from vehicle-treated WT mice. Data represent the mean ± SD from 5 experiments. *P < .05; **P < .01; ***P < .001.
To determine if MMPs are involved in degrading core 1 O-glycan–deficient PDPN in vivo, P7 EHC C1galt1−/− or WT mice were treated for 6 days with daily IP injections of GM6001 or vehicle control, and then sacrificed at P14. GM6001 did not cause any significant physiological changes in the treated mice, as GM6001- and vehicle-treated EHC C1galt1−/− mice exhibited comparable peripheral blood cell counts and growth curves (supplemental Table 1; supplemental Figure 9). Additionally, GM6001- and vehicle-treated EHC C1galt1−/− mice expressed similar levels of Tn antigen (Figure 4C), indicating equivalent efficiency of C1galt1 deletion. Immunofluorescent staining of cryosections of intestines showed increased PDPN levels in lymphatic vessels from GM6001-treated but not vehicle-treated EHC C1galt1−/− mice. Furthermore, flow cytometry demonstrated that PDPN surface levels on isolated mesenteric LECs from GM6001-treated EHC C1galt1−/− mice was significantly higher than that on LECs of vehicle-treated EHC C1galt1−/− mice (Figure 4D). These data indicate that MMPs are responsible for proteolytic degradation of PDPN on LECs in EHC C1galt1−/− mice.
Sialylated O-glycans protect PDPN from proteolysis in the CLP sepsis model
Sialic acids are the common capping structure of O-glycans. Using the WT and C1galt1−/− ECs with similar levels of PDPN (Figure 5A) that was not altered by sialidase treatment, the glycan profile showed that limax flavus agglutinin (LFA) (to sialic acids) and maackia amurensis lectin II (MAL-II) (to α2,3 sialylated glycans), but not peanut agglutinin (PNA) (to desialylated core 1 O-glycans), sambucus nigra agglutinin (to α2,6 sialylated GalNAc-Ser/Thr), helix pomatia agglutinin (HPA) (to Tn antigen), and ricinus communis agglutinin (RCA120) (to LacNAc), interacted strongly with WT PDPN (Figure 5B). Sialidase treatment decreased reactivity of WT PDPN with LFA and MAL-II, and increased that with PNA and RCA120. These results indicate that WT PDPN is modified by α2,3 sialylated core 1 and core 1-derived O-glycans (supplemental Figure 10). In contrast, core 1 O-glycan–deficient PDPN with or without sialidase treatment reacted only with HPA, indicating that core 1 O-glycan–deficient PDPN presents terminal GalNAc without detectable sialylation. These data suggest that lack of sialylation or core 1 O-glycosylation, or both in combination, may contribute to the increased PDPN degradation on C1galt1−/− LECs. To test this, we pretreated the WT ECs with sialidases and then incubated them with MMP-2/9 or trypsin (Figure 5C and supplemental Figure 11). Surface level of PDPN on sialidase-treated WT ECs was reduced by MMP-2/9 or trypsin, but not as much as on the C1galt1−/− ECs. These reductions were rescued with GM6001 or benzamidine. This result supports that lack of sialylation is sufficient to cause significant degradation of PDPN.
Sialic acids play an important role in the protection of PDPN from proteolytic degradation. (A) Flow cytometric analyses of cell surface PDPN on WT or C1galt1−/− ECs with or without sialidase treatment. Cells were incubated with hamster anti-PDPN antibody 8.1.1, followed by PE-conjugated anti-hamster IgG. (B) Glycan profile of WT PDPN-eGFP or core 1 O-glycan–deficient PDPN-GFP with or without sialidase treatment. WT PDPN-eGFP or core 1 O-glycan–deficient PDPN-eGFP was purified by mouse anti-GFP monoclonal antibody agarose beads from WT ECs or C1galt1−/− ECs. The fluorescence intensity of anti-PDPN antibody or lectin binding is indicated with bars. Lectin specificities are as follows: LFA (sialic acid); MAL-II (Siaα2-3Galβ1-3 ± [Siaα2-6]GalNAc); sambucus nigra agglutinin (Siaα2-6Gal or Siaα2-6GalNAc); PNA (desialylated Galβ1-3GalNAc [core 1]); HPA (terminal GalNAc [Tn]); and RCA120 (desialylated Galβ1-4GlcNAc [LacNAc]). The data represent the mean ± SD from 3 experiments. (C) WT ECs were pretreated with sialidase and then incubated in buffer (as a control), or 2 μg/ml activated MMP-2/MMP-9 with or without 100 μM GM6001. The cells were stained with anti-PDPN antibody for flow cytometry. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 experiments. (D) Sialidase activity was measured using RCA120 reactivity with fetuin treated by culture medium, the lymph from sham, the lymph from CLP mice, or the lymph from CLP mice with 300 μg/ml of sialidase inhibitor at 37°C for 2 hours. The fluorescence intensity of RCA120 binding is indicated with bars. The data represent the mean ± SD from 3 experiments. (E) WT ECs were incubated in culture medium (as a control), lymph from sham, or lymph from CLP mice with or without 100 μM GM6001, 100 μM GM6001 and 2 mM PMSF, or 300 μg/ml sialidase inhibitor. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 experiments. (F, top) Flow cytometric analyses of cell surface PDPN on isolated mesenteric LECs from mice treated by sham with vehicle, CLP with vehicle, CLP with GM6001, or CLP with sialidase inhibitor. PDPN expression was analyzed on CD31+/Lyve-1+ LEC population. (F, bottom) Quantification of the percentages of PDPN+ LEC numbers (left) and percentages of MFIs of PDPN on LECs (right) are shown. MFIs of PDPN on LECs from CLP mice were normalized to the MFI of PDPN on LECs from sham mice. Data represent the mean ± SD from 4 experiments. *P < .05; **P < .01; ***P < .001. ND, not detected.
Sialic acids play an important role in the protection of PDPN from proteolytic degradation. (A) Flow cytometric analyses of cell surface PDPN on WT or C1galt1−/− ECs with or without sialidase treatment. Cells were incubated with hamster anti-PDPN antibody 8.1.1, followed by PE-conjugated anti-hamster IgG. (B) Glycan profile of WT PDPN-eGFP or core 1 O-glycan–deficient PDPN-GFP with or without sialidase treatment. WT PDPN-eGFP or core 1 O-glycan–deficient PDPN-eGFP was purified by mouse anti-GFP monoclonal antibody agarose beads from WT ECs or C1galt1−/− ECs. The fluorescence intensity of anti-PDPN antibody or lectin binding is indicated with bars. Lectin specificities are as follows: LFA (sialic acid); MAL-II (Siaα2-3Galβ1-3 ± [Siaα2-6]GalNAc); sambucus nigra agglutinin (Siaα2-6Gal or Siaα2-6GalNAc); PNA (desialylated Galβ1-3GalNAc [core 1]); HPA (terminal GalNAc [Tn]); and RCA120 (desialylated Galβ1-4GlcNAc [LacNAc]). The data represent the mean ± SD from 3 experiments. (C) WT ECs were pretreated with sialidase and then incubated in buffer (as a control), or 2 μg/ml activated MMP-2/MMP-9 with or without 100 μM GM6001. The cells were stained with anti-PDPN antibody for flow cytometry. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 experiments. (D) Sialidase activity was measured using RCA120 reactivity with fetuin treated by culture medium, the lymph from sham, the lymph from CLP mice, or the lymph from CLP mice with 300 μg/ml of sialidase inhibitor at 37°C for 2 hours. The fluorescence intensity of RCA120 binding is indicated with bars. The data represent the mean ± SD from 3 experiments. (E) WT ECs were incubated in culture medium (as a control), lymph from sham, or lymph from CLP mice with or without 100 μM GM6001, 100 μM GM6001 and 2 mM PMSF, or 300 μg/ml sialidase inhibitor. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 experiments. (F, top) Flow cytometric analyses of cell surface PDPN on isolated mesenteric LECs from mice treated by sham with vehicle, CLP with vehicle, CLP with GM6001, or CLP with sialidase inhibitor. PDPN expression was analyzed on CD31+/Lyve-1+ LEC population. (F, bottom) Quantification of the percentages of PDPN+ LEC numbers (left) and percentages of MFIs of PDPN on LECs (right) are shown. MFIs of PDPN on LECs from CLP mice were normalized to the MFI of PDPN on LECs from sham mice. Data represent the mean ± SD from 4 experiments. *P < .05; **P < .01; ***P < .001. ND, not detected.
To determine the pathological relevance of this finding, we used a CLP sepsis model, which has increased sialidase activity in serum.26 Firstly, lymph was collected from CLP or sham mice. Consistent with the published data, increased sialidase activity was detected in the CLP lymph, which was blocked by the sialidase inhibitor but not in the sham lymph (Figure 5D). The CLP lymph reduced PDPN levels on WT ECs, which was blocked by GM6001 (Figure 5E). A combination of PMSF with GM6001 or sialidase inhibitor alone blocked this PDPN reduction more effectively. Furthermore, we found that the levels of PDPN on LECs from CLP mice were significantly reduced compared with that on LECs from sham mice (Figure 5F). This reduction was rescued by an IP injection of GM6001 or a sialidase inhibitor. These data indicate that sialylation of O-glycans is important in preventing PDPN from proteolytic degradation in a sepsis model.
Endothelial PDPN requires sialylated core 1 O-glycans to interact with platelets under flow
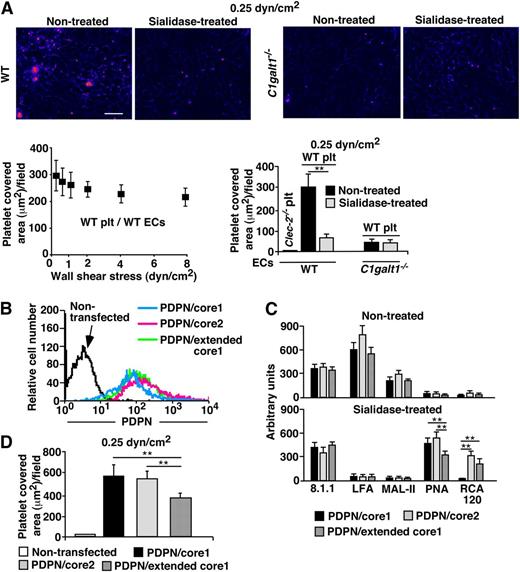
Previous studies using transfected CHO cells show that sialylated core 1 O-glycans on PDPN is important for interacting with platelet CLEC-2.7,14 Unlike ECs, CHO cells do not express extended core 1 or core 2 O-glycans. Lymphatic PDPN interacts with platelet CLEC-2 to regulate lymphatic functions, likely under venous flow conditions.8,27 Therefore, it remains to be determined whether sialylated core 1 O-glycans are required for endothelial PDPN to bind to platelet CLEC-2 under physiological flow. Flow chamber data revealed stable adhesion and aggregation of WT but not Clec-2−/− platelets on the WT ECs under shear stresses similar to the venous flow conditions.12,28 Sialidase treatment markedly reduced this platelet adhesion (Figure 6A). In contrast, only a few platelets adhered on the C1galt1−/− ECs with or without sialidase treatment at 0.25 dyn/cm2 of shear stress. These data suggest that sialylated core 1 and/or core 1-derived O-glycans are important for endothelial PDPN to interact with platelets.
Sialylated core 1 O-glycans of PDPN plays an important role in platelet adhesion on ECs under flow. (A) PHK26 labeled platelets from WT or platelet-specific Clec-2−/− mice were perfused and allowed to accumulate on WT or C1galt1−/− ECs with or without sialidase treatment under shear stress of 0.25 dyn/cm2 for 10 minutes. After changing to platelet-free buffer, fluid shear stress was increased every 30 seconds. Dual bright field and fluorescence images of platelet adhesion and aggregation on ECs under shear stress of 0.25 dyn/cm2 are shown. Scale bar, 20 μm. The images are representative of 3 independent experiments. ECs in bright fields are shown as blue color, and fluorescent PHK-labeled platelets are shown as red color. The data quantify the covered platelet area on each EC under the indicated shear stresses and represent the mean ± SD from 3 experiments. (B) Flow cytometric analyses of cell surface PDPN on CHO cells transfected with PDPN-eGFP and control vector (PDPN/core1), PDPN-eGFP and core2GlcNAcT (PDPN/core2), or PDPN-eGFP and core1βGlcNAcT (PDPN/extended core1). Cells were incubated with hamster anti-PDPN antibody 8.1.1, followed by PE-conjugated anti-hamster IgG. (C) Glycan profile of PDPN/core1, PDPN/core2, or PDPN/extended core 1 with or without sialidase treatment is shown. PDPN-eGFP from different CHO cell lines was captured on agarose beads conjugated with mouse anti-GFP monoclonal antibody. Glycan profile of PDPN was analyzed based on lectin binding. See Figure 5 for lectin specificities. The fluorescence intensity of anti-PDPN antibody or lectin binding is indicated in the bar graphs. The data represent the mean ± SD from 3 experiments. (D) PDPN/core1, PDPN/core2, or PDPN/extended core1 CHO cells were cultured in 35 mm dishes, and PHK26-labeled platelets from WT were perfused and allowed to accumulate on CHO cells under shear stress of 0.25 dyn/cm2 for 10 minutes. The data quantify the covered platelet area on CHO cells under the indicated shear stress, and represent the mean ± SD from 3 experiments. **P < .01. Plt, platelets.
Sialylated core 1 O-glycans of PDPN plays an important role in platelet adhesion on ECs under flow. (A) PHK26 labeled platelets from WT or platelet-specific Clec-2−/− mice were perfused and allowed to accumulate on WT or C1galt1−/− ECs with or without sialidase treatment under shear stress of 0.25 dyn/cm2 for 10 minutes. After changing to platelet-free buffer, fluid shear stress was increased every 30 seconds. Dual bright field and fluorescence images of platelet adhesion and aggregation on ECs under shear stress of 0.25 dyn/cm2 are shown. Scale bar, 20 μm. The images are representative of 3 independent experiments. ECs in bright fields are shown as blue color, and fluorescent PHK-labeled platelets are shown as red color. The data quantify the covered platelet area on each EC under the indicated shear stresses and represent the mean ± SD from 3 experiments. (B) Flow cytometric analyses of cell surface PDPN on CHO cells transfected with PDPN-eGFP and control vector (PDPN/core1), PDPN-eGFP and core2GlcNAcT (PDPN/core2), or PDPN-eGFP and core1βGlcNAcT (PDPN/extended core1). Cells were incubated with hamster anti-PDPN antibody 8.1.1, followed by PE-conjugated anti-hamster IgG. (C) Glycan profile of PDPN/core1, PDPN/core2, or PDPN/extended core 1 with or without sialidase treatment is shown. PDPN-eGFP from different CHO cell lines was captured on agarose beads conjugated with mouse anti-GFP monoclonal antibody. Glycan profile of PDPN was analyzed based on lectin binding. See Figure 5 for lectin specificities. The fluorescence intensity of anti-PDPN antibody or lectin binding is indicated in the bar graphs. The data represent the mean ± SD from 3 experiments. (D) PDPN/core1, PDPN/core2, or PDPN/extended core1 CHO cells were cultured in 35 mm dishes, and PHK26-labeled platelets from WT were perfused and allowed to accumulate on CHO cells under shear stress of 0.25 dyn/cm2 for 10 minutes. The data quantify the covered platelet area on CHO cells under the indicated shear stress, and represent the mean ± SD from 3 experiments. **P < .01. Plt, platelets.
To determine whether extended core 1 or core 2 O-glycans are important for PDPN function (supplemental Figure 10), we co-transfected CHO cells with complementary DNAs encoding PDPN-eGFP and empty vector (PDPN/core 1), PDPN-eGFP and core2GlcNAcT (PDPN/core 2), or PDPN-eGFP and core1βGlcNAcT (PDPN/extended core 1). These CHO cells express similar levels of PDPN (Figure 6B). Glycan analysis using lectins as probes (supplemental Figure 10) showed that LFA and MAL-II interacted with PDPN/core 1, PDPN/core 2, and PDPN/extended core 1 (Figure 6C), which was abolished by sialidase treatment. In contrast, PNA bound to all forms of desialylated PDPN, and RCA120 only reacted with desialylated PDPN/core 2 and PDPN/extended core 1 but not PDPN/core 1. These results support that PDPN is primarily modified by a sialylated core 1 structure. Consistent with this, WT platelets interacted similarly with CHO cells expressing PDPN/core 1 and with PDPN/core 2 (Figure 6D). Interestingly, extended core 1 O-glycosylation appeared to reduce PDPN interaction with WT platelets, as there was a modest reduction of platelet binding to CHO cells with PDPN/extended core 1. Taken together, sialylated core 1 O-glycosylation of PDPN on ECs is sufficient to interact with platelet CLEC-2 under flow.
Discussion
We previously reported that core 1 O-glycosylation is critical for the expression of functional PDPN during the development of lymphatic vessels.8 However, the mechanism of how O-glycosylation regulates PDPN function and expression was unclear. In this study, we demonstrate that sialylated core 1 O-glycosylation is essential for the stability of PDPN, by protecting it from proteolytic degradation by MMPs in the lymph, and for platelet adhesion and aggregation on LECs under flow conditions.
The protective role of O-glycosylation against proteolysis of modified proteins has been previously demonstrated in vitro.19,21 However, the nature of the proteases and whether this function is important in vivo have not been elucidated. Our results show that core 1 O-glycan–deficient PDPN is susceptible to different types of proteases that have predicted cleavage sites in the extracellular domain of PDPN, such as serine protease, trypsin and elastase, cysteine protease calpain-2, and MMP2/9. Interestingly, most of the predicted protease cleavage sites are located in close proximity to the potential O-glycosylation sites of PDPN (Figure 3C). Mucin-type O-glycoproteins typically have a rod conformation.29 The first α-linked GalNAc is essential for the extended conformation.30 Because the α-GalNAc remains attached to the peptide backbone of PDPN in the absence of core 1 O-glycosylation, it is unlikely that the increased proteolytic degradation of core 1-deficient PDPN is due to its conformational changes that favor the accessibility of proteases to their cleavage sites.
Negatively charged sialic acids are common capping structures in O-glycans and are reported to be important for protecting glycoproteins from proteolysis.31-33 Adding exogenous GalNAc to ldl-D CHO cells, which cannot synthesize uridine diphosphate-Gal/uridine diphosphate-GalNAc, generates sialylated and more stable proteins against proteolysis compared with the proteins without GalNAc, suggesting a protective mechanism of sialylated GalNAc.19,34 Interestingly, core 1 O-glycan–deficient PDPN is not sialylated. The lack of sialylation may contribute to increased proteolysis of core 1 O-glycan–deficient PDPN. Supporting this idea, our data showed that desialylation significantly increased susceptibility of PDPN to the proteases, but not as much as lack of core 1 O-glycan (supplemental Figure 11). Core 1 O-glycans can be extended to form extended core 1 or core 2 O-glycans. Mice lacking these glycan structures35,36 or lacking core 3-derived O-glycans (including core 4),37 do not exhibit lymphatic vascular defects that resemble mice without core 1 O-glycans. Together, these data indicate that sialylation and basic core 1 structure cooperate together to protect PDPN from proteolytic degradation. Desialylation of glycans are known to contribute to increased clearance of platelets and von Willebrand factor in bacteria-mediated sepsis models.38 Consistently, increased sialidase activity in serum is reported in sepsis patients39 and in rodent CLP sepsis models.26 Our data showed the increased sialidase in the lymph from CLP mice, which caused desialylation and promoted degradation of PDPN on LECs primarily by MMPs in the lymph. Although it remains to be further determined, these results imply that desialylation of O-glycans leads to abnormal lymphatic function in pathological conditions, such as sepsis.
The lymph of humans and rodents contains proteases, including MMP-2/9, and serine proteases thrombin, plasmin, and factor X.40,41 Therefore, although our in vitro and in vivo analyses showed that MMPs are a major form of proteases in degrading core 1 O-glycan–deficient or desialylated PDPN, other proteases such as serine proteases, may also be involved in the regulation of PDPN levels.42-44
PDPN induces platelet aggregation by interacting with CLEC-2 on platelets.12,45 Previous studies using transfected CHO cells and the glioblastoma cell line6 demonstrated that sialylated core 1 O-glycans on PDPN is required for platelet aggregation.7,14 Our results, using Lyve-1–positive ECs show that sialylated core 1 structure is present on WT PDPN, consistent with published data.6 Platelets adhered and aggregated on WT, but not on core 1 O-glycan–deficient PDPN, under shears. Our studies further demonstrate that extended core 1 or core 2 O-glycans are not required for PDPN to interact with platelet CLEC-2. These results support that sialylated core 1 O-glycan on endothelial PDPN is sufficient to mediate stable platelet adhesion and aggregation under shears.
Recently, PDPN-mediated platelet activation has been found to be critical in maintaining the integrity of high endothelial venules in the lymph node and for lymphovenous hemostasis in the adult mouse.13,27 Therefore, O-glycosylation in regulating the function and expression of PDPN is important not only for the development of an independent lymphatic vascular system, but also for the maintenance of the established blood-lymphatic vascular systems throughout life. Abnormal glycosylation of PDPN may contribute to pathogenesis of diseases such as sepsis, as demonstrated in the CLP model.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the National Institutes of Health (National Institute of General Medical Sciences: GM103441; and National Heart, Lung, and Blood Institute: HL085607, HL093242, and HL118676), Department of Defense (W81XWH-11-1-0226), Oklahoma Center for the Advancement of Science (HR13-160 and HR13-020), National Natural Science Foundation of China (30928010 and 31400692), Jiangsu Provincial Special Program of Medical Science (BL2012005), Jiangsu Province’s Key Medical Center (ZX201102), and the American Heart Association (SDG7410022).
Authorship
Contribution: Y.P., T.Y., B.H., and J.M.M. performed research; Y.P., T.Y., J.F., P.M-D., X.C., C.R., R.P.M., C.W., K.D., H.C., and L.X. analyzed data; and T.Y. and L.X. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lijun Xia, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, MS #45, 825 N.E. 13th St, Oklahoma City, OK 73104; e-mail: Lijun-Xia@omrf.org; and Tadayuki Yago, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: Tadayuki-Yago@omrf.org.

![Figure 5. Sialic acids play an important role in the protection of PDPN from proteolytic degradation. (A) Flow cytometric analyses of cell surface PDPN on WT or C1galt1−/− ECs with or without sialidase treatment. Cells were incubated with hamster anti-PDPN antibody 8.1.1, followed by PE-conjugated anti-hamster IgG. (B) Glycan profile of WT PDPN-eGFP or core 1 O-glycan–deficient PDPN-GFP with or without sialidase treatment. WT PDPN-eGFP or core 1 O-glycan–deficient PDPN-eGFP was purified by mouse anti-GFP monoclonal antibody agarose beads from WT ECs or C1galt1−/− ECs. The fluorescence intensity of anti-PDPN antibody or lectin binding is indicated with bars. Lectin specificities are as follows: LFA (sialic acid); MAL-II (Siaα2-3Galβ1-3 ± [Siaα2-6]GalNAc); sambucus nigra agglutinin (Siaα2-6Gal or Siaα2-6GalNAc); PNA (desialylated Galβ1-3GalNAc [core 1]); HPA (terminal GalNAc [Tn]); and RCA120 (desialylated Galβ1-4GlcNAc [LacNAc]). The data represent the mean ± SD from 3 experiments. (C) WT ECs were pretreated with sialidase and then incubated in buffer (as a control), or 2 μg/ml activated MMP-2/MMP-9 with or without 100 μM GM6001. The cells were stained with anti-PDPN antibody for flow cytometry. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 experiments. (D) Sialidase activity was measured using RCA120 reactivity with fetuin treated by culture medium, the lymph from sham, the lymph from CLP mice, or the lymph from CLP mice with 300 μg/ml of sialidase inhibitor at 37°C for 2 hours. The fluorescence intensity of RCA120 binding is indicated with bars. The data represent the mean ± SD from 3 experiments. (E) WT ECs were incubated in culture medium (as a control), lymph from sham, or lymph from CLP mice with or without 100 μM GM6001, 100 μM GM6001 and 2 mM PMSF, or 300 μg/ml sialidase inhibitor. The bar graph indicates the percentage of MFI of cell surface PDPN levels against that of control. The data represent the mean ± SD from 3 experiments. (F, top) Flow cytometric analyses of cell surface PDPN on isolated mesenteric LECs from mice treated by sham with vehicle, CLP with vehicle, CLP with GM6001, or CLP with sialidase inhibitor. PDPN expression was analyzed on CD31+/Lyve-1+ LEC population. (F, bottom) Quantification of the percentages of PDPN+ LEC numbers (left) and percentages of MFIs of PDPN on LECs (right) are shown. MFIs of PDPN on LECs from CLP mice were normalized to the MFI of PDPN on LECs from sham mice. Data represent the mean ± SD from 4 experiments. *P < .05; **P < .01; ***P < .001. ND, not detected.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/24/10.1182_blood-2014-04-572107/4/m_3656f5.jpeg?Expires=1768810802&Signature=e6mLDP4RKiwcTEDKE09AdDceOP7SswknaLFe113etYUJm-GNgpPS-YP8VeOT2gUsYOUptDFMIqrNMSbFsaFH-bVODkoJbSFBcJfU7g51VVzYbNu9RLXPXht2dGe3cm3s-I5xkdcNepGwj6kEVcRZKeNPeBEZxOvrQWtVxD-mxUTvntreZjRjxfM4spheppCa64dfnhJfb2YQ2tJZw00TSe-W4dKYRu6rbMN9STJ8kVoyLhXULSDzHknYh8oATeoo3~B1Y7GNdna5NmSmj5MDsEgIxQQyHjhiG6nkhuUocXPZbHM1H7Kk~xoHe7m7xYUmV4mjbRKis4rGJK9TZUQU9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal