Key Points
Human IRAK-4 and MyD88 deficiencies impair T-independent IgM production, including IgM recognizing bacterial antigens.
T-independent IgM impairment by IRAK-4 and MyD88 deficiencies is linked to inadequacy of the IgM+IgD+CD27+ B-cell subset.
Abstract
IRAK-4 and MyD88 deficiencies impair interleukin 1 receptor and Toll-like receptor (TLR) signaling and lead to heightened susceptibility to invasive bacterial infections. Individuals with these primary immunodeficiencies have fewer immunoglobulin M (IgM)+IgD+CD27+ B cells, a population that resembles murine splenic marginal zone B cells that mount T-independent antibody responses against bacterial antigens. However, the significance of this B-cell subset in humans is poorly understood. Using both a 610 carbohydrate array and enzyme-linked immunosorbent assay, we found that patients with IRAK-4 and MyD88 deficiencies have reduced serum IgM, but not IgG antibody, recognizing T-independent bacterial antigens. Moreover, the quantity of specific IgM correlated with IgM+IgD+CD27+ B-cell frequencies. As with mouse marginal zone B cells, human IgM+CD27+ B cells activated by TLR7 or TLR9 agonists produced phosphorylcholine-specific IgM. Further linking splenic IgM+IgD+CD27+ B cells with production of T-independent IgM, serum from splenectomized subjects, who also have few IgM+IgD+CD27+ B cells, had reduced antibacterial IgM. IRAK-4 and MyD88 deficiencies impaired TLR-induced proliferation of this B-cell subset, suggesting a means by which loss of this activation pathway leads to reduced cell numbers. Thus, by bolstering the IgM+IgD+CD27+ B-cell subset, IRAK-4 and MyD88 promote optimal T-independent IgM antibody responses against bacteria in humans.
Introduction
Autosomal recessive IRAK-4 and MyD88 deficiencies lead to marked susceptibility to bacterial infections, most frequently Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa.1-3 Defects in either IRAK-4 or MyD88 impair activation of nuclear factor κB and mitogen-activated protein kinase, leading to defective induction of downstream cytokines in response to interleukin-1 receptor (IL-1R) and Toll-like receptor (TLR) ligands.1,2,4 MyD88 also interacts with transmembrane activator and CAML interactor to functionally cooperate with TLRs.5,6 IRAK-4– and MyD88-dependent signaling appears vital for early childhood immunity to pyogenic bacteria, but appears less important with increasing age, because no deaths in these patients have been reported beyond the age of 8 years and no invasive infections have been seen after age 14.3,7 The immunological basis of this age-related clinical improvement has yet to be explained. Patients with defects in IRAK-4 and MyD88 have greatly reduced frequency of circulating immunoglobulin M (IgM)+IgD+CD27+ B cells,8 a B-cell subset that resembles splenic marginal zone B cells and may be activated in the absence of T-cell help.9,10 In mice, splenic marginal zone B cells respond rapidly and robustly to phosphorylcholine (PC),11 a polar head group of the pneumococcal cell wall12 producing anti-PC IgM within 3 days of challenge with S. pneumoniae.13,14 Absence of this early PC-specific IgM response heightens susceptibility to pneumococcal infection in mice,15-19 and impaired antibody responses to PC have also been associated with pneumococcal infections and bacteremia in humans.20,21
Using a multiplex carbohydrate array and assays for selected T-independent bacterial antigens, we demonstrate here that the sera of patients with IRAK-4 and MyD88 deficiencies are globally deficient in IgM antibacterial antibodies. In addition, we found that serum levels of specific IgM responses correlated with the proportion of IgM+IgD+CD27+ B cells in peripheral blood, both in subjects with defective IRAK-4 and MyD88 and in splenectomized patients who experience an acquired depletion of these cells.22 We further found that TLR7 or TLR9 stimulation of cells from control subjects induced preferential proliferation of IgM+IgD+CD27+ B cells, suggesting a mechanistic link between defects in MyD88 and IRAK-4, loss of IgM+IgD+CD27+ B cells, and important antibacterial antibodies. Together, these data provide evidence that IRAK-4 and MyD88 deficiencies lead to inadequacy of IgM+IgD+CD27+ B-cell numbers, diminished serum T–independent IgM responses, and early susceptibility to pyogenic infection.
Patients, materials, and methods
Patients and controls
Eleven IRAK-4– and MyD88-deficient patients of aged 1 to 30 years were studied (Table 1). Disease controls included 14 subjects with histories of either invasive pneumococcal disease (IPD; meningitis or sepsis) or recurrent pyogenic infections, but not with mutations or defects in IRAK-4 or MyD88 (Table 2). Matching healthy control samples were derived from blood remaining from routine laboratory testing of healthy children, with these samples deidentified so that only date of birth and gender were known. Twenty-one splenectomized subjects (supplemental Table 1, available on the Blood Web site) were also studied, all of whom had been vaccinated against pneumococcus on study enrollment, and had not received steroids, immunoglobulin, rituximab, or other immunosuppressive medications within 6 months of enrollment. Splenectomy controls were healthy consenting adults aged 26 to 64 years who had not received a previous pneumococcal vaccination. This work was approved by the institutional review boards of Icahn School of Medicine at Mount Sinai and Necker Hospital, and informed consent was obtained from each patient or parent in accordance with the Declaration of Helsinki.
Characteristics of IRAK-4– and MyD88-deficient patients
| Age (y) . | Gender . | Mutation . | Invasive infection history . | IgG (mg/dL) . | IgA (mg/dL) . | IgM (mg/dL) . | IgE (kU/L) . | Tetanus (IU/mL) . | S. pneumoniae (μg/mL) . | Isohemagglutinin . | IgM+ IgD+ CD27+ (% of B cells) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IRAK-4−/− | |||||||||||
| 1 | F | L360X/L360X | Bacterial meningitis (twin with P. aeruginosa) | 680 | 171 | 116 | ND | ND | ND | ND | ND |
| 3 | F | M1V/1188+520A>G | P. aeruginosa | 1690 | 109 | 98 | 257 | 1.15 | 2.04 | ND | 0.8 |
| 3 | F | Y430X/1126-1G>T | S. pneumoniae, S. agalactiae, S. sonnei | 1120 | 119 | 159 | 6 | 0.58 | 8.11 | ND | ND |
| 9 | M | 1189-1G > T/1188+520A>G | S. pneumoniae | 1360 | 242 | 52 | 187 | 0.59 | <0.3 | 1/2 | 2.4 |
| 12 | M | Q293X/Q293X | S. pneumoniae | 1390 | 165 | 89 | NA | >0.1 | 2.0 | 1/64, 1/32 | 6 |
| 17 | M | Q293X/large de novo del | S. pneumoniae, S. aureus | 1440 | 88 | 64 | 11 | 0.47 | <0.3 | ND | 1.4 |
| 18 | M | R12C/831+5G>T | S. aureus, P. aeruginosa | 1280 | 102 | 138 | 180 | ND | ND | ND | 3.7 |
| 30 | F | Q293X/Q293X | S. pneumoniae, S. sonnei | 1670 | 110 | 190 | 400 | 0.06 | >0.3 | 1/2 | ND |
| MyD88−/− | |||||||||||
| 1 | F | E65del/E65del | S. pneumoniae, S. aureus, H. influenzae, M. catarrhalis | 379* | 22* | 32* | ND | ND | ND | ND | ND |
| 1 | M | E65del/E65del | P. aeruginosa | 620 | 52 | 93 | ND | ND | 0.9 | ND | ND |
| 11 | F | R209C/R209C | S. pneumoniae, Salmonella spp | 1250 | 136 | 69 | 10.4 | NA | 3.85 | 1/16 | 9 |
| Normal reference values | |||||||||||
| 1-3 y | 335-896 | 27-122 | 58-153 | <40 | >0.1 | >0.3 | >1/16 | 5-12 | |||
| 3-7 y | 549-1154 | 41-157 | 54-155 | <60 | >0.1 | >0.3 | >1/16 | 5-12 | |||
| >7 y | 655-1278 | 70-344 | 50-209 | <150 | >0.1 | >0.3 | >1/16 | 5-12 | |||
| Age (y) . | Gender . | Mutation . | Invasive infection history . | IgG (mg/dL) . | IgA (mg/dL) . | IgM (mg/dL) . | IgE (kU/L) . | Tetanus (IU/mL) . | S. pneumoniae (μg/mL) . | Isohemagglutinin . | IgM+ IgD+ CD27+ (% of B cells) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IRAK-4−/− | |||||||||||
| 1 | F | L360X/L360X | Bacterial meningitis (twin with P. aeruginosa) | 680 | 171 | 116 | ND | ND | ND | ND | ND |
| 3 | F | M1V/1188+520A>G | P. aeruginosa | 1690 | 109 | 98 | 257 | 1.15 | 2.04 | ND | 0.8 |
| 3 | F | Y430X/1126-1G>T | S. pneumoniae, S. agalactiae, S. sonnei | 1120 | 119 | 159 | 6 | 0.58 | 8.11 | ND | ND |
| 9 | M | 1189-1G > T/1188+520A>G | S. pneumoniae | 1360 | 242 | 52 | 187 | 0.59 | <0.3 | 1/2 | 2.4 |
| 12 | M | Q293X/Q293X | S. pneumoniae | 1390 | 165 | 89 | NA | >0.1 | 2.0 | 1/64, 1/32 | 6 |
| 17 | M | Q293X/large de novo del | S. pneumoniae, S. aureus | 1440 | 88 | 64 | 11 | 0.47 | <0.3 | ND | 1.4 |
| 18 | M | R12C/831+5G>T | S. aureus, P. aeruginosa | 1280 | 102 | 138 | 180 | ND | ND | ND | 3.7 |
| 30 | F | Q293X/Q293X | S. pneumoniae, S. sonnei | 1670 | 110 | 190 | 400 | 0.06 | >0.3 | 1/2 | ND |
| MyD88−/− | |||||||||||
| 1 | F | E65del/E65del | S. pneumoniae, S. aureus, H. influenzae, M. catarrhalis | 379* | 22* | 32* | ND | ND | ND | ND | ND |
| 1 | M | E65del/E65del | P. aeruginosa | 620 | 52 | 93 | ND | ND | 0.9 | ND | ND |
| 11 | F | R209C/R209C | S. pneumoniae, Salmonella spp | 1250 | 136 | 69 | 10.4 | NA | 3.85 | 1/16 | 9 |
| Normal reference values | |||||||||||
| 1-3 y | 335-896 | 27-122 | 58-153 | <40 | >0.1 | >0.3 | >1/16 | 5-12 | |||
| 3-7 y | 549-1154 | 41-157 | 54-155 | <60 | >0.1 | >0.3 | >1/16 | 5-12 | |||
| >7 y | 655-1278 | 70-344 | 50-209 | <150 | >0.1 | >0.3 | >1/16 | 5-12 | |||
Reference values as published.3
M. catarrhalis, Moraxella catarrhalis; NA, not available; ND, not determined; P. aeruginosa, Pseudomonas aeruginosa; S. agalactiae, Streptococcus agalactiae; S. sonnei, Shigella sonnei.
Patient <6 mo of age when laboratory values were drawn.
Characteristics of patients with invasive or recurrent bacterial infection
| Age (y) . | Infection history . | IgG (mg/dL) . | IgA (mg/dL) . | IgM (mg/dL) . | IgM+IgD+CD27+ (%) . | IgM−IgD−CD27+ (%) . |
|---|---|---|---|---|---|---|
| Invasive | ||||||
| 2.5 | Pneumococcal meningitis and recurrent skin infection | 944 | 41 | 81 | NA | NA |
| 3 | Pneumococcal meningitis, Haemophilus influenzae type B meningitis, and myocarditis | 703 | 63 | 102 | 4.7 | 8 |
| 3 | Pneumococcal meningitis and recurrent skin infection | 539 | 32 | 77 | 5.9 | 2.1 |
| 3 | Recurrent pneumococcal meningitis | 719 | 40 | 41 | 8.8 | 2.3 |
| 5 | Pneumococcal sepsis and recurrent pneumonia | 889 | 43 | 61 | 1.59 | 6.3 |
| 7 | Pneumococcal meningitis and sepsis | 573 | 131 | 88 | 9.2 | 8 |
| 10 | Pneumococcal meningitis | 1850 | 76 | 100 | ND | ND |
| Recurrent | ||||||
| 8 | RPI | 1060 | 66 | 123 | 10.7 | 6.5 |
| 4 | Recurrent pyogenic skin infections | 1010 | 41 | 81 | 7.3 | 5.6 |
| 6 | Recurrent pneumonia, recurrent sinusitis and otitis | 889 | 58 | 101 | 11 | 10.8 |
| 4 | Recurrent pneumonia, recurrent sinusitis and otitis | 924 | 67 | 74 | 5.9 | 2.8 |
| 2 | Recurrent pneumonia | 437 | 34 | 60 | 6 | 2.8 |
| 30 | Recurrent sinusitis | 1160 | 0 | 135 | ND | ND |
| 11 | Recurrent pneumonia and otitis | 959 | 276 | 111 | ND | ND |
| Normal reference values | ||||||
| 1-3 y | 335-896 | 27-122 | 58-153 | 5-12 | 2-10 | |
| 3-7 y | 549-1154 | 41-157 | 54-155 | 5-12 | 2-10 | |
| >7 y | 655-1278 | 70-344 | 50-209 | 5-12 | 2-10 | |
| Age (y) . | Infection history . | IgG (mg/dL) . | IgA (mg/dL) . | IgM (mg/dL) . | IgM+IgD+CD27+ (%) . | IgM−IgD−CD27+ (%) . |
|---|---|---|---|---|---|---|
| Invasive | ||||||
| 2.5 | Pneumococcal meningitis and recurrent skin infection | 944 | 41 | 81 | NA | NA |
| 3 | Pneumococcal meningitis, Haemophilus influenzae type B meningitis, and myocarditis | 703 | 63 | 102 | 4.7 | 8 |
| 3 | Pneumococcal meningitis and recurrent skin infection | 539 | 32 | 77 | 5.9 | 2.1 |
| 3 | Recurrent pneumococcal meningitis | 719 | 40 | 41 | 8.8 | 2.3 |
| 5 | Pneumococcal sepsis and recurrent pneumonia | 889 | 43 | 61 | 1.59 | 6.3 |
| 7 | Pneumococcal meningitis and sepsis | 573 | 131 | 88 | 9.2 | 8 |
| 10 | Pneumococcal meningitis | 1850 | 76 | 100 | ND | ND |
| Recurrent | ||||||
| 8 | RPI | 1060 | 66 | 123 | 10.7 | 6.5 |
| 4 | Recurrent pyogenic skin infections | 1010 | 41 | 81 | 7.3 | 5.6 |
| 6 | Recurrent pneumonia, recurrent sinusitis and otitis | 889 | 58 | 101 | 11 | 10.8 |
| 4 | Recurrent pneumonia, recurrent sinusitis and otitis | 924 | 67 | 74 | 5.9 | 2.8 |
| 2 | Recurrent pneumonia | 437 | 34 | 60 | 6 | 2.8 |
| 30 | Recurrent sinusitis | 1160 | 0 | 135 | ND | ND |
| 11 | Recurrent pneumonia and otitis | 959 | 276 | 111 | ND | ND |
| Normal reference values | ||||||
| 1-3 y | 335-896 | 27-122 | 58-153 | 5-12 | 2-10 | |
| 3-7 y | 549-1154 | 41-157 | 54-155 | 5-12 | 2-10 | |
| >7 y | 655-1278 | 70-344 | 50-209 | 5-12 | 2-10 | |
RPI, recurrent pyogenic infection.
Carbohydrate array
The 610 carbohydrate array from the Consortium for Functional Glycomics (CFG) (functionalglycomics.org) was prepared from amine functionalized carbohydrate structures covalently coupled in microarrays to N-hydroxysuccinimide–derivatized microscope slides as previously described.23 Versions 5.0 and 5.1 of the CFG carbohydrate array both were used, with 1 carbohydrate present on version 5.0 (#56) that was not present on version 5.1 and thus was omitted from analysis. In total, 610 carbohydrates in replicates of 6 were analyzed. Carbohydrates on the CFG array expressed by S. aureus or S. pneumoniae were identified by using the substructure search on the Bacterial Carbohydrate Structure Database (http://csdb.glycoscience.ru/bacterial/). IgG and IgM binding to individual carbohydrates on the array were measured using fluorescently labeled detection antibodies and expressed as relative fluorescent units (RFU). The Glyco Pattern online program (glycopattern.emory.edu) was used to compare data between different versions of the carbohydrate array and to establish the RFU threshold used to define binding and nonbinding carbohydrates.24
Specific antibacterial antibodies
Nunc MaxiSorp 96-well plates were coated with 10 μg/mL purified capsular polysaccharide from S. pneumoniae serotypes 4, 14, and 23F (ATCC); 20 μg/mL S. pneumoniae teichoic acid (also known as the pneumococcal common antigen or C-polysaccharide) (Statens Serum Institut); 10 μg/mL PC (Sigma); 20 μg/mL S. aureus teichoic acid (Sigma); or 1 μL/100 μL IgM coating antibody (Bethyl) overnight at 4°C. Plates were then washed with phosphate-buffered saline (PBS)/0.05% Tween 20, blocked with at least 30 minutes at room temperature with PBS/0.05% Tween 20/0.5% bovine serum albumin (BSA), washed again, then incubated with serum or supernatant from cell culture for 90 minutes at room temperature. Plates were then washed and incubated with horseradish peroxidase (HRP)-conjugated IgM or IgG detection antibodies (Bethyl) for 90 minutes. After final washes, plates were developed with TMB substrate (BD Biosciences), with 2N H2SO4 (Sigma) added after being fully developed. Absorbance of wells was measured at an optical density (OD) of 450 nm by a POLARstar Ω plate reader (BMG Labtech). Human serum with a known concentration of immunoglobulin isotypes (Bethyl) was used as a reference standard for conversion of OD values to an arbitrary concentration labeled as arbitrary units.
Antibacterial antibody complement activation
Nunc MaxiSorp 96-well plates were coated with antigens, blocked, and washed as described previously, then incubated with serum supplemented with 10% human complement (Sigma) for 1 hour at 37%. Serum was then transferred to a 96-well plate coated with antibody against C3a/C3a des Arg (Abcam) and incubated for 90 minutes at room temperature. The plate was washed and then incubated with a biotinylated different clone of anti-C3a/C3a des Arg antibody (Abcam) for 90 minutes at room temperature. After washing, the plates were incubated with streptavidin-HRP (Abcam) for 90 minutes. Plates were washed again and then developed with TMB substrate (BD Biosciences); absorbance was measured at an OD of 450 nm.
TLR activation and cell culture
Peripheral blood mononuclear cells (PBMCs) were incubated in 96-well plates (BD Biosciences) at a concentration of 5 × 105 cells/200 μL in RPMI 1640/antibiotic-antimycotic (Gibco) and 10% fetal bovine serum (Atlanta Biologicals) and stimulated with 1 μg/mL Clo97 (TLR7/8 agonist, InvivoGen), 0.5 μg/mL ODN 2006 (TLR9 agonist, InvivoGen), 0.5 μg/mL CD40L, 100 ng/mL IL-4, 50 ng/mL IL-10 (all from R&D Systems), or 10 ng/mL of IL-21 (Cell Signaling) for 5 days. To examine cell proliferation, PBMCs were suspended in prewarmed PBS with 0.5% BSA and labeled with 5 µM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) for 5 minutes at room temperature, washed with 0.5% BSA/PBS, and cultured for 5 days. To isolate B cells before culture, they were sorted into naive CD27− and memory CD27+ subsets (Aria, BD Biosciences) and incubated in 96-well plates (BD Biosciences) at a concentration of 1 × 105 cells/200 μL in RPMI 1640 supplemented with 1% antibiotic-antimycotic (Gibco), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Gibco), sodium pyruvate (Gibco), and 10% fetal bovine serum (Atlanta Biologicals) with a stimulate of 0.25 μg/mL Clo97 (TLR7/8 agonist, InvivoGen), 0.3 μg/mL ODN 2006 (TLR9 agonist, InvivoGen), or 150 ng/mL CD40L with 10 ng/mL IL-21 for 7 days. Immunoglobulin production was measured from cell culture supernatants as mentioned. Flow cytometry analysis of B-cell subsets was conducted using multicolor flow cytometer FACSCalibur (BD Biosciences) and FloJo computer software (Tree Star). The fluorescein-conjugated antibodies used were CD19-PC5 (Beckman Coulter), CD27-FITC (Dako), IgD-PE (BD Pharmingen), and IgM-DyLight649 (Jackson ImmunoResearch).
Splenectomized subjects
We determined the number of peripheral blood IgM+IgD+CD27+ B cells in the blood of 21 adult subjects who had undergone splenectomy for autoimmune thrombocytopenia or hereditary spherocytosis (supplemental Table 1). We also examined antipneumococcal antibody responses in these subjects 28 days after vaccination with 23-valent pneumococcal polysaccharide vaccine (PPV23). IgG and IgM antibody responses were measured and compared with 7 age-matched healthy controls.
Statistical analysis
Nonparametric Mann-Whitney testing was used to assess the statistical significance for 2 group comparisons of cell culture experiments. Differences between groups for all other values, other than the carbohydrate array, were assessed using the nonparametric Kruskal-Wallis test. If the Kruskal-Wallis test yielded a P value of less than .05, differences between subject groups were assessed using Dunn’s multiple comparisons. Carbohydrate array results were rank-transformed and analyzed by 1-way analysis of variance. If an analysis of variance F test yielded a P value of less than .05, differences between groups were assessed using Dunnett’s test, which adjusts for multiple comparisons. The Spearman rank correlation test was used to measure the significance of correlations between IgM+IgD+CD27+ B cells and specific IgM responses. Calculations were made using Prism software (GraphPad). A P value less than .05 was considered significant.
Results
IRAK-4– and MyD88-deficient patients have impaired T-independent IgM responses
Quantitative levels of total serum IgG and IgM are not reduced in patients with defects in IRAK-4 or MyD88, and lack of IgG antibody has not been consistently observed (Table 1).3 In this study, only 1 of 6 patients had an absent IgG response to tetanus vaccine, 2 of 8 patients had deficient IgG response to pneumococcal vaccination, and 1 of 4 patients had reduced IgM allo-hemagglutinins directed against erythrocyte AB antigens.3 Because these patients have had recurrent infections with encapsulated bacteria, we more broadly examined their anti-carbohydrate antibody responses. To that end, we used a 610 carbohydrate array to assess global carbohydrate-specific Ig responses in serum of 8 IRAK-4– and 3 MyD88-deficient patients in comparison with the serum of 2 control groups: 3 patients with a history of severe pneumococcal infection but without mutations in IRAK-4, MyD88, or other genetic defects (including asplenia, complement deficiency, and primary antibody deficiency) and 8 age-matched healthy controls.
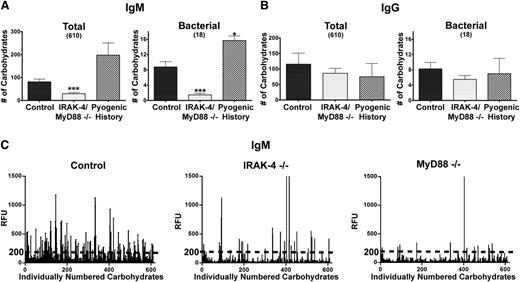
IRAK-4 and MyD88 deficiencies share identical patterns of infection susceptibility and natural history, and both have reduced IgM+IgD+CD27+ B cells and function jointly in a shared biochemical pathway.3,8 As we found no significant difference in numbers of array carbohydrates bound by IgM (P = .92) or IgG (P = .28) between those with IRAK-4 and MyD88 deficiencies, these patients were grouped together for further analysis because of the small sample sizes resulting from the rarity of these diseases. Serum IgM from both IRAK-4– or MyD88-deficient patients bound significantly fewer carbohydrates on the CFG array compared with healthy controls or patients with pyogenic infections, both overall and for 18 carbohydrates identified through the Bacterial Carbohydrate Structure Database as being expressed by S. aureus or S. pneumoniae (Figure 1A; supplemental Table 2). In contrast, carbohydrate-specific serum of the IgG isotype did not differ between IRAK-4– and MyD88-deficient patients as well as the other infection groups on either measure (Figure 1B). Representative carbohydrate arrays demonstrate the broad reduction in anti-carbohydrate antibodies seen in IRAK-4– or MyD88-deficient patients, as reflected in the paucity of array carbohydrates bound by serum IgM (Figure 1C). Also illustrated is the RFU threshold used to define specific binding to carbohydrates, determined to be 200 RFU using the Glyco Pattern program (glycopattern.emory.edu).
IRAK-4 and MyD88 deficiencies impair T-independent IgM responses. Quantity of total and bacterial array carbohydrates bound by serum (A) IgM and (B) IgG. Control (n = 8), IRAK-4 deficient (n = 8), MyD88 deficient (n = 3). (C) Example CFG array results with dotted line illustrating RFU cutoff for bound carbohydrates. Bar represents mean; whiskers represent standard error of the mean. ***P < .001.
IRAK-4 and MyD88 deficiencies impair T-independent IgM responses. Quantity of total and bacterial array carbohydrates bound by serum (A) IgM and (B) IgG. Control (n = 8), IRAK-4 deficient (n = 8), MyD88 deficient (n = 3). (C) Example CFG array results with dotted line illustrating RFU cutoff for bound carbohydrates. Bar represents mean; whiskers represent standard error of the mean. ***P < .001.
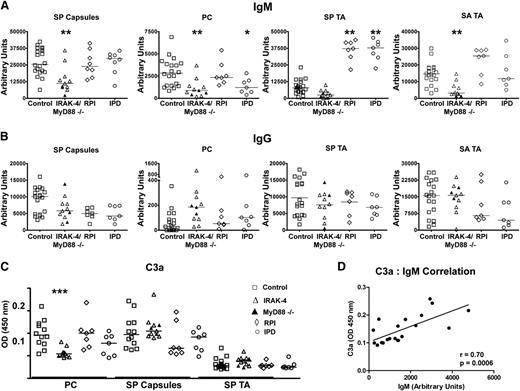
Having found defects in IgM recognizing carbohydrates, including bacterial carbohydrates, in serum of IRAK-4– and MyD88-deficient patients, we next wanted to more carefully study antibacterial IgM in these subjects. As such, we quantified IgM recognizing specific T-independent bacterial antigens in the serum of these patients compared with 14 patients with similar bacterial infection histories and 19 age-matched healthy controls (Table 2). Control patients with infection histories included 7 with a history of S. pneumoniae meningitis and/or sepsis (IPD) and 7 with a history of recurrent bacterial cutaneous or respiratory infections but no invasive infections (RPI). Enzyme-linked immunosorbent assay (ELISA) was used to quantify serum Ig for T-independent antigens derived from S. aureus and S. pneumoniae, namely S. pneumoniae capsular polysaccharides (SP capsules), PC, and S. pneumoniae and S. aureus teichoic acid (SP TA, SA TA). Serum of patients with IRAK-4 or MyD88 deficiencies had a highly significant reduction in IgM recognizing SP capsules, PC, and SA TA as well a trend toward reduced IgM recognizing SP TA relative to controls (Figure 2A). IPD patients also had significantly reduced PC-specific IgM, yet both IPD and RPI groups had elevated SP TA–specific IgM relative to IRAK-4– or MyD88-deficient patients as well as controls. In contrast, as had been noted for the anti-carbohydrate array, subjects with IRAK-4 or MyD88 defects had no impairment of antigen-specific IgG for any of these antigens (Figure 2B).
IRAK-4 and MyD88 deficiencies impair antibacterial IgM responses. Levels of (A) IgM and (B) IgG specific for SP capsules, PC, SP TA, and SA TA. MyD88-deficient subjects (▲). (C) Amount of C3a released from serum after incubation with designated pneumococcal antigens. Control (n = 8), IRAK-4 deficient (n = 8), MyD88 deficient (n = 3). Data are results from 1 of 2 replicate experiments. (D) Levels of C3a detected in serum after incubation with PC correlate with levels of PC-specific IgM. P value represents the Spearman rank correlation test. *P < .05, **P < .01, ***P < .001.
IRAK-4 and MyD88 deficiencies impair antibacterial IgM responses. Levels of (A) IgM and (B) IgG specific for SP capsules, PC, SP TA, and SA TA. MyD88-deficient subjects (▲). (C) Amount of C3a released from serum after incubation with designated pneumococcal antigens. Control (n = 8), IRAK-4 deficient (n = 8), MyD88 deficient (n = 3). Data are results from 1 of 2 replicate experiments. (D) Levels of C3a detected in serum after incubation with PC correlate with levels of PC-specific IgM. P value represents the Spearman rank correlation test. *P < .05, **P < .01, ***P < .001.
Because we saw reduction in antibacterial IgM, but not antibacterial IgG in IRAK-4– and MyD88-deficient patients, we tested whether there was an impairment of humoral immunity caused by this specific IgM deficiency. Furthermore, we wanted to confirm whether any impairment could be compensated by corresponding levels of a specific IgG antibody. Thus, we tested serum for C3 activation upon antibody binding for 3 T-independent pneumococcal antigens: 1 in which specific IgM levels were markedly higher than that of IgG (PC), 1 in which specific IgM levels in serum were comparable to that of IgG (SP capsules), and 1 that is known to poorly fix complement by any antibody isotype (SP TA).25-27 Defective complement fixation was found in IRAK-4– and MyD88-deficient subjects only for PC (Figure 2C), illustrating that specific IgM deficiency impairs humoral immunity in cases where compensatory IgG levels are not present. As further evidence that reduced antibody levels lead to reduced specific complement activation, correlation was found between levels of PC-specific IgM and C3a levels in serum incubated with PC (Figure 2D).
Specific IgM responses correlate with levels IgM+IgD+CD27+ B cells
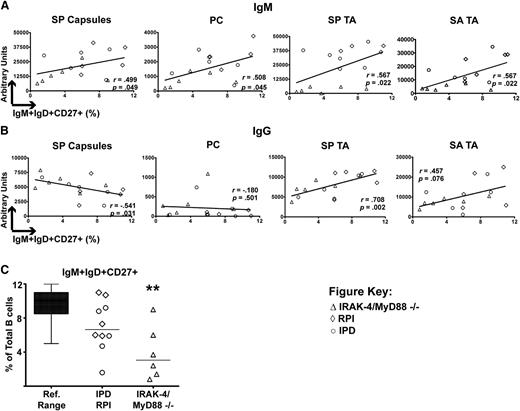
Given these hints at the importance of serum IgM, we next focused upon the IgM+IgD+CD27+ B-cell subset because these are a candidate source of such antibodies and a population known to be reduced in IRAK-4– and MyD88-deficient patients.8-10 To determine whether this population might be responsible for secreting antibacterial IgM, we compared the frequency of circulating IgM+IgD+CD27+ B cells to serum levels of antibacterial IgM in subjects for which these values were available (IRAK-4– and MyD88-deficient, IPD, and RPI patients). Indeed, we found that increased levels of serum IgM against SP capsules, PC, SP TA, and SA TA all correlated with greater proportions of IgM+IgD+CD27+ B cells (Figure 3A). In contrast, there was not a positive correlation between IgG recognizing SP capsules or PC and frequencies of IgM+IgD+CD27+ B cells, and only a trend toward correlation for SA TA (Figure 3B). Thus, the correlation between IgM+IgD+CD27+ B cells and T-independent IgM responses was found for more antigens than that of IgM+IgD+CD27+ B cells and IgG. Of note, frequencies of IgM+IgD+CD27+ B cells in IRAK-4– and MyD88-deficient subjects were significantly lower than the reference range of this lymphocyte subset (Figure 3C).
Specific IgM responses correlate with IgM+IgD+CD27+ B cells. (A) Serum IgM recognizing SP capsules, PC, SP TA, and SA TA have positive correlation with percentage of IgM+IgD+CD27+ B cells. (B) Only serum IgG for SP capsules positively correlates with IgM+IgD+CD27+ B cells. P value represents the Spearman rank correlation test. (C) IgM+IgD+CD27+ B cells as a percentage of total B cells. **P < .01.
Specific IgM responses correlate with IgM+IgD+CD27+ B cells. (A) Serum IgM recognizing SP capsules, PC, SP TA, and SA TA have positive correlation with percentage of IgM+IgD+CD27+ B cells. (B) Only serum IgG for SP capsules positively correlates with IgM+IgD+CD27+ B cells. P value represents the Spearman rank correlation test. (C) IgM+IgD+CD27+ B cells as a percentage of total B cells. **P < .01.
Splenectomy impairs specific IgM in correlation with levels of IgM+IgD+CD27+ B cells
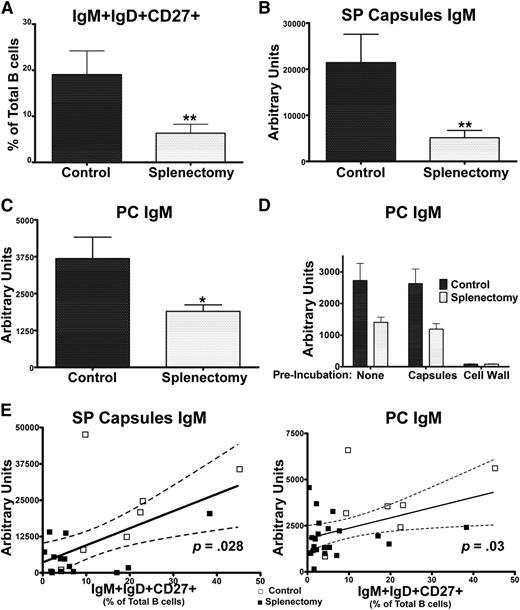
To further examine the relationship between IgM+IgD+CD27+ B cells and antibacterial antibody responses, we examined a group of splenectomized subjects. As previously reported,9,28,29 these subjects had reduced circulating IgM+IgD+CD27+ B cells (Figure 4A). Importantly, this patient cohort allows us to assess the outcome of reductions in this B-cell subset independent of IRAK-4 and MyD88 defects. After PPV23 immunization, which contains pneumococcal PC as well as capsular polysaccharides,30,31 splenectomized subjects had significantly reduced IgM that recognized SP capsules (Figure 4B) and PC (Figure 4C) as compared with controls, mirroring reductions seen in IRAK-4– and MyD88-deficient patients, as measured by ELISA. To confirm the specificity of IgM recognizing PC, the anti-PC ELISA was repeated using serum preincubated with either SP capsules, which do not contain PC, or with SP cell wall, which does, demonstrating high specificity of these antibodies (Figure 4D). In addition, as had been seen in the IRAK-4– and MyD88-deficient patients, increased frequencies of IgM+IgD+CD27+ cells correlated with higher levels of serum IgM recognizing SP capsules and PC in splenectomized subjects (Figure 4E).
Splenectomy impairs specific IgM in correlation with levels of IgM+IgD+CD27+ B cells. (A) IgM+IgD+CD27+ percentage of total B cells. (B) Serum IgM binding SP capsules and (C) PC. (D) S. pneumoniae cell wall, but not capsules, blocks binding of PC-specific IgM. (E) Serum IgM recognizing SP capsules and PC correlate with percentage of IgM+IgD+CD27+ B cells. Controls (□); splenectomized subjects (▪). P value represents the Spearman rank correlation test. Dotted line indicates 95% confidence interval. Control (n = 7); splenectomy (n = 21). Results derived from 1 of 2 replicate experiments. Bar represents mean; whiskers represent standard error of the mean. *P < .05, **P < .01.
Splenectomy impairs specific IgM in correlation with levels of IgM+IgD+CD27+ B cells. (A) IgM+IgD+CD27+ percentage of total B cells. (B) Serum IgM binding SP capsules and (C) PC. (D) S. pneumoniae cell wall, but not capsules, blocks binding of PC-specific IgM. (E) Serum IgM recognizing SP capsules and PC correlate with percentage of IgM+IgD+CD27+ B cells. Controls (□); splenectomized subjects (▪). P value represents the Spearman rank correlation test. Dotted line indicates 95% confidence interval. Control (n = 7); splenectomy (n = 21). Results derived from 1 of 2 replicate experiments. Bar represents mean; whiskers represent standard error of the mean. *P < .05, **P < .01.
PC-specific IgM is produced by human CD27+ B cells upon IRAK-4– and MyD88-dependent TLR stimulation
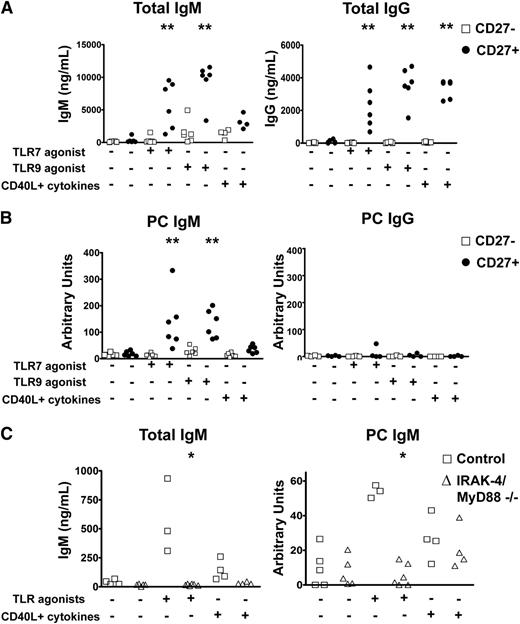
Having correlated the IgM+IgD+CD27+ B-cell subset to T-independent IgM responses, we next wished to determine whether these cells were indeed a source of these antibodies. To that end, we sorted CD27+ and CD27− cells of 4 to 6 healthy donors and cultured them with TLR7 or TLR9 agonists as IRAK-4– and MyD88-dependent activation signals, or, for comparison, CD40L with cytokines (IL-4 and IL-10 or IL-21). As expected, CD27+ B cells produced higher levels of IgM and IgG in response to all stimuli relative to CD27− B cells (Figure 5A). PC-specific IgM was produced exclusively by CD27+ B cells, with higher levels induced by TLR stimulations relative to CD40L and cytokines (Figure 5B). In contrast, almost no PC-specific IgG was detectable in any of these cultures. When comparable experiments were carried out with samples from IRAK-4– and MyD88-deficient subjects, CD27+ B cells failed to produce PC-specific IgM after culture with TLR7 and TLR9 agonists, but showed similar amounts of PC-specific IgM when activated by CD40L and cytokines (Figure 5C).
PC-specific IgM is produced by human CD27+ B cells upon IRAK-4– and MyD88-dependent TLR stimulation. (A) CD27+ B cells produce higher levels of total IgM and IgG when stimulated with TLR7 and TLR9 agonists. (B) PC-specific antibodies are produced by CD27+IgM+ B cells. Data from sorted PBMC samples derived from 6 unique individuals. (C) IRAK-4 and MyD88 deficiencies impair production of total and PC-specific IgM in response to TLR7 and TLR9 stimulation of PBMC cultures. Control (n = 4), IRAK-4/MyD88 deficient (n = 4). *P < .05, **P < .01, ***P < .001.
PC-specific IgM is produced by human CD27+ B cells upon IRAK-4– and MyD88-dependent TLR stimulation. (A) CD27+ B cells produce higher levels of total IgM and IgG when stimulated with TLR7 and TLR9 agonists. (B) PC-specific antibodies are produced by CD27+IgM+ B cells. Data from sorted PBMC samples derived from 6 unique individuals. (C) IRAK-4 and MyD88 deficiencies impair production of total and PC-specific IgM in response to TLR7 and TLR9 stimulation of PBMC cultures. Control (n = 4), IRAK-4/MyD88 deficient (n = 4). *P < .05, **P < .01, ***P < .001.
Impaired proliferation of IgM+IgD+CD27+ B cells in IRAK-4 and MyD88 deficiencies
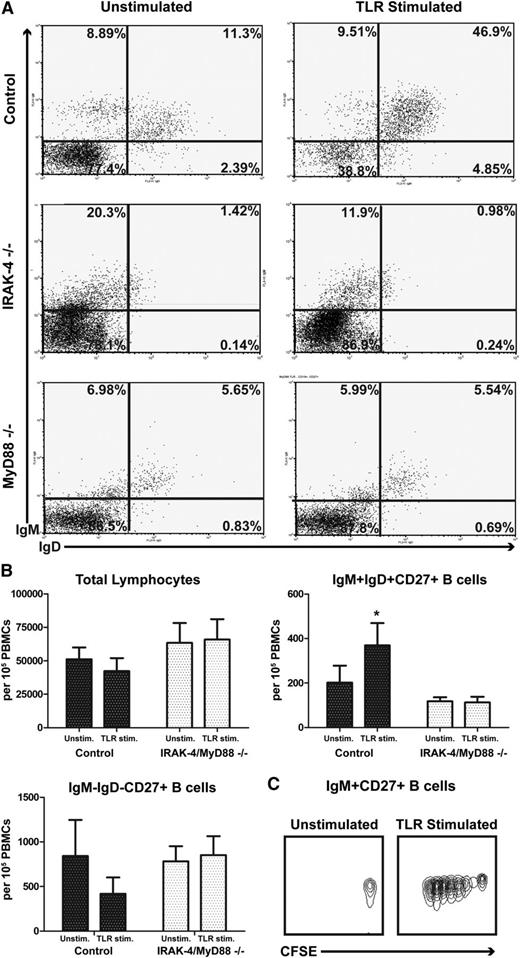
Having seen that TLR stimulation could induce T-independent antibacterial IgM antibodies, and that this IgM arose largely from CD27+IgM+ cells, which are considerably depleted in subjects with defects in IRAK-4 and MyD88, we investigated whether IRAK-4– and MyD88-dependent signals might specifically enhance proliferation of IgM+IgD+CD27+ B cells. After culture with TLR7 or TLR9 agonists, IgM+IgD+CD27+ B cells of control subjects preferentially expanded in PBMC cultures, doing so more extensively than IgM−IgD− CD27+ B cells and thereby leading to a selective enrichment of this B-cell subset (Figure 6A-B). As expected, proliferation of IgM+IgD+CD27+ B cells was substantially impaired in IRAK-4– and MyD88-deficient patients who cannot signal through TLR7 or TLR9, with frequencies of these cells largely unchanged by stimulation. Both IRAK-4– and MyD88-deficient samples had a similar absence of IgM+IgD+CD27+ B-cell enrichment after culture with TLR agonists. IgM+CD27+ B cells from control samples proliferate in response to TLR agonists, as demonstrated by dilution with CFSE dye (Figure 6C).
IRAK-4 and MyD88 deficiencies impair IgD+IgM+CD27+ B-cell proliferation. (A) TLR agonists enrich IgM+IgD+CD27+ B cells in culture of control PBMCs, but not IRAK-4– or MyD88-deficient PBMCs. Gated on CD19+, CD27+. Representative of 4 experiments. (B) Quantification of total lymphocytes, IgM+IgD+ and IgM−IgD− CD27+ B cells with and without TLR stimulation. Data from 4 individuals per group. Box represents mean; whiskers represent standard error of mean. (C) Dilution of CFSE after TLR stimulation. Representative of 3 experiments. *P < .05.
IRAK-4 and MyD88 deficiencies impair IgD+IgM+CD27+ B-cell proliferation. (A) TLR agonists enrich IgM+IgD+CD27+ B cells in culture of control PBMCs, but not IRAK-4– or MyD88-deficient PBMCs. Gated on CD19+, CD27+. Representative of 4 experiments. (B) Quantification of total lymphocytes, IgM+IgD+ and IgM−IgD− CD27+ B cells with and without TLR stimulation. Data from 4 individuals per group. Box represents mean; whiskers represent standard error of mean. (C) Dilution of CFSE after TLR stimulation. Representative of 3 experiments. *P < .05.
Discussion
Susceptibility to bacterial infections is often indicative of primary antibody deficiency.32 Anti-carbohydrate antibodies are particularly important in immunity against bacteria that use carbohydrate capsules.33 Although total Ig levels are not reduced in patients with IRAK-4 and MyD88 deficiencies, impairment of pneumococcal-specific IgG has been reported in some,34,35 but has not been correlated with the occurrence of invasive bacterial infections.3 More recently, it has been reported that circulating IgM+IgD+CD27+ B cells, which produce T-independent IgM responses,10 are depleted in these patients, suggesting that the loss of this B-cell subset may be clinically important.8 In mice, marginal zone B cells produce protective T-independent antibacterial IgM, but a similar role for the corresponding IgM+IgD+CD27+ B cells in humans has yet to be defined.36
We found that IRAK-4– and MyD88-deficient patients have broadly impaired IgM, but not IgG, responses against T-independent antigens—in particular to the carbohydrate antigens expressed by S. aureus and S. pneumoniae, organisms to which these patients are particularly susceptible.3,37 IgM uses its pentameric structure to potently bind carbohydrates, fixes complement on bacterial surfaces more effectively than IgG,38,39 and is rapidly produced after TLR signaling without the requirement of T-cell help.40,41 Exemplifying its role against encapsulated bacteria, IgM antibodies provide protection against pneumococcus in both humans and murine models.22,42-44 IgM appears to contribute to respiratory host defense against Haemophilus influenzae and protect against the development of bronchiectasis in patients with hypogammaglobulinemia.45,46
Curiously, we noted that 3 array carbohydrates were recognized quite effectively by serum IgM from patients with IRAK-4 or MyD88 deficiencies, at levels comparable to controls. These carbohydrates are all derivatives of the Forssman antigen (supplemental Figure 1A). These are not inherent high-background carbohydrates on this array (David F. Smith, Emory University, personal communication). The absence of impaired IgM responses against the Forssman antigen was also confirmed by ELISA, which showed no impairment of IgM specific for this antigen in IRAK-4 and MyD88 deficiencies (supplemental Figure 1B). Thus, the Forssman antigen appears to be a unique carbohydrate for which IgM responses can be mounted without IRAK-4 and MyD88. The Forssman antigen is a heterophile glycoprotein expressed by animals and enteric bacteria that is a target of natural IgM antibodies associated with hyperacute rejection in xenotransplantation.47 The significance of this finding has yet to be elucidated.
Defective complement fixation was noted in patients with IRAK-4 and MyD88 deficiencies only for an antigen for which specific IgM impairment was not compensated for by IgG. This result suggests that the combination of age-related impairment of anti-carbohydrate IgG coupled with a genetic impairment of T-independent IgM may underlie heightened susceptibility to pyogenic infections. IgG responses against certain pneumococcal serotypes may not be effectively mounted until after age 5, and anti-carbohydrate IgG may not reach mature levels until as late as teenage years.48,49 Moreover, because IgM appears to have a specialized role in combating bloodstream infections,50-56 the early propensity of invasive infections in IRAK-4 and MyD88 deficiencies may also be explained by impairment of T-independent IgM. Along these lines, it is notable that IgG replacement therapy is used to treat patients with IRAK-4 and MyD88 deficiencies.3
It has been suggested that marginal zone B cells are contained within the human IgM+IgD+CD27+ B-cell subset.9 Marginal zone B cells are an important source of rapid antibacterial antibody responses in mice, particularly of the IgM isotype.13,14 Some of the results from our studies suggest marginal zone B cell–like features of human IgM+IgD+ B cells. First, we noted that IRAK-4 and MyD88 deficiencies impair rapid TLR7- and TLR9-mediated IgM production by IgM+CD27+ B cells.41,57-63 Additionally, we found the PC-specific antibody to be derived from human CD27+ B cells and predominantly of the IgM isotype, as with the PC-specific IgM produced by murine marginal zone B cells.13-15 Finally, antibacterial IgM in the serum positively correlated with the numbers of IgM+IgD+CD27+ B cells similarly in splenectomy and IRAK-4 and MyD88 deficiencies, suggesting that this B-cell subset and its T-independent antibacterial IgM responses may be derived from the spleen-like murine marginal zone B cells.13-15,62 However, our results are limited to indirect evidence of marginal zone-like B-cell features of human IgM+IgD+CD27+ B cells because no splenic B cells could be studied.
The association between IgM+IgD+CD27+ B cells and TLR signals requires further investigation. It is notable that reduction of IgM+IgD+CD27+ B cells via splenectomy or IRAK-4 and MyD88 deficiencies resulted in a similar impairment of T-independent IgM responses. An explanation for the reduction in IgM+IgD+CD27+ B cells as a result of IRAK-4 and MyD88 deficiencies could be that tonic signals provided through IRAK-4– and MyD88-dependent TLRs, perhaps from commensal bacterial organisms, are required to maintain optimal numbers of these cells.64 Our studies focused upon TLR7 and TLR9 because of the expression of these TLRs on B cells and availability of potent agonists. It is interesting to note that UNC-93B–deficient patients, who also have an impairment of both TLR7 and TLR9 signaling,65 do not have a reduction of peripheral IgM+IgD+CD27+ B cells.8 Accordingly, receptors that are IRAK-4– and MyD88-dependent but UNC-93B–independent, such as transmembrane activator and CAML interactor or TLR10,37,66 might be responsible for this B-cell subset impairment in IRAK-4– and MyD88-deficient patients. Furthermore, cells expressing IRAK-4– and MyD88-dependent receptors other than B lymphocytes may be required for sending proliferative signals to IgM+IgD+CD27+ B cells.
Our study furthers the accumulation of provocative data asserting the IgM+IgD+CD27+ B-cell subset as an important contributor to antibacterial host defense. Although we show antibacterial IgM deficiency in IRAK-4– and MyD88-deficient patients, pervasive defects in cytokine and cell activation pathways are also well characterized1-4 and may further compound immune impairment in these patients. More investigation is necessary to confirm the antibacterial potential of IgM+IgD+CD27+ B cells, prove this circulating B-cell subset is derived from the splenic marginal zone, and determine whether this mode of antibacterial immunity can be harnessed therapeutically.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Montserrat Cols and Jean-Claude Weill for critically reading this manuscript and offering helpful suggestions, David F. Smith for assistance with the Consortium for Functional Glycomics array, and Laureen Ojalvo for thoughtful discussion; Drs L. Marodi, I. Reisli, H. Chapel, S. Zitnik, J.C. Krause, C. Hoarrau, and J. Vasconcelos for sending blood from the patients with IRAK-4 and MyD88 deficiencies; Maya Chrabieh for technical help; and all health care providers participating in the management of these patients as well as the patients themselves for their participation.
This work was supported by the Thrasher Research Fund Early Career Award, Baxter-Clinical Immunology Society Senior Fellowship Award, the Jeffrey Modell Foundation, Fondation pour la Recherche Médicale (DMI20091117320), the March of Dimes (1-FY12-440), the National Center for Research Resources and the National Center for Advancing Sciences of the National Institutes of Health (grant 8UL1TR000043), National Institutes of Health (grants AI048693, AI061093, and T32 GM007280), the David S. Gottesman Immunology Chair, the St. Giles Foundation, the Rockefeller University, INSERM, Paris Descartes University, and the Icahn School of Medicine at Mount Sinai.
Authorship
Contribution: P.J.M., N.S., S.B., and L.R. performed experiments; P.J.M., J.R.O., and E.B. did statistical analyses; J.B.B., X.B., J.-L.C., I.M., and C.P. provided patient samples and clinical data; P.J.M., A.C., C.P., and C.C.-R. designed the research; and P.J.M. wrote the initial draft of the manuscript. All authors analyzed and interpreted the data, contributed toward critical revision of the manuscript, and approved the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charlotte Cunningham-Rundles, Division of Clinical Immunology, Department of Medicine, Icahn School of Medicine at Mount Sinai, 1425 Madison Ave, New York, NY 10029; e-mail: charlotte.cunningham-rundles@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal