Key Points
Inhibition of Akt signaling promotes generation of superior tumor-reactive T cells with stem cell–like properties.
Adoptive transfer of Akt-inhibited tumor-reactive T cells results in superior antitumor effect.
Abstract
Effective T-cell therapy against cancer is dependent on the formation of long-lived, stem cell–like T cells with the ability to self-renew and differentiate into potent effector cells. Here, we investigated the in vivo existence of stem cell–like antigen-specific T cells in allogeneic stem cell transplantation (allo-SCT) patients and their ex vivo generation for additive treatment posttransplant. Early after allo-SCT, CD8+ stem cell memory T cells targeting minor histocompatibility antigens (MiHAs) expressed by recipient tumor cells were not detectable, emphasizing the need for improved additive MiHA-specific T-cell therapy. Importantly, MiHA-specific CD8+ T cells with an early CCR7+CD62L+CD45RO+CD27+CD28+CD95+ memory-like phenotype and gene signature could be expanded from naive precursors by inhibiting Akt signaling during ex vivo priming and expansion. This resulted in a MiHA-specific CD8+ T-cell population containing a high proportion of stem cell–like T cells compared with terminal differentiated effector T cells in control cultures. Importantly, these Akt-inhibited MiHA-specific CD8+ T cells showed a superior expansion capacity in vitro and in immunodeficient mice and induced a superior antitumor effect in intrafemural multiple myeloma–bearing mice. These findings provide a rationale for clinical exploitation of ex vivo–generated Akt-inhibited MiHA-specific CD8+ T cells in additive immunotherapy to prevent or treat relapse in allo-SCT patients.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a potentially curative therapy for high-risk hematologic malignancies.1-3 The therapeutic effect is attributed to the graft-versus-tumor (GVT) response, during which donor-derived CD8+ T cells become activated by recipient minor histocompatibility antigens (MiHAs) presented on dendritic cells (DCs).4 Subsequently, these activated CD8+ T cells expand and differentiate into effector cells that kill MiHA-positive tumor cells. However, persistence and recurrence of the malignant disease is often observed, indicating that insufficient GVT immunity is induced. This suboptimal antitumor CD8+ T-cell response might be a result of lack of robust, long-lived, high-quality memory T-cell function.5
The current strategy for enhancing GVT responses after allo-SCT is the adoptive transfer of polyclonal donor T cells known as donor lymphocyte infusion.3,6,7 However, this nonselected donor T-cell product contains a limited number of T-cell precursors, recognizing hematopoietic-restricted MiHAs and/or tumor-associated antigens that are able to contribute to the GVT effect. Moreover, it contains T cells that recognize polymorphic antigens expressed by healthy host tissues, thereby causing graft-versus-host disease (GVHD), the major cause of morbidity and mortality after allo-SCT.8 Therefore, adoptive T-cell therapy with purified and expanded donor CD8+ T cells that are selective for MiHAs restricted to the hematopoietic system could lead to a more efficacious GVT effect without promoting GVHD. Nevertheless, current ex vivo expansion protocols for generating MiHA-specific CD8+ T cells concomitantly results in effector memory T cells (Tem) and terminal differentiated effectors.9,10 Other studies suggest that these subsets comprise the least potent cells for adoptive T-cell therapy because of their poor proliferative capacity, lack of ability to maintain robust responses over time, and vulnerability to exhaustion.11-13 Stem cell memory T cells (Tscm) cells and central memory T cells (Tcm) would be more favorable populations for MiHA-based adoptive immunotherapy after allo-SCT. Especially Tscm cells have enhanced proliferation, self-renewal, and multipotency.12,14 Importantly, these long-lived Tscm cells have the plasticity to differentiate into Tcm and Tem, which rapidly acquire effector functions after antigenic re-exposure. In addition, a recent publication by Graef et al15 showed the stem cell properties of Tcm cells. Because the proliferative and differentiation capacity of these subsets is retained, they provide a better chance to generate long-term antitumor T-cell responses and ultimately eliminate all residual malignant cells.11
The PI3K-Akt-mTOR pathway plays an important role in regulating CD8+ T-cell metabolism and differentiation.16 Upon signaling via the T-cell receptor, costimulatory molecules, and cytokine receptors, the PI3K-Akt pathway is activated, resulting in downstream activation of the mammalian target of rapamycin (mTOR) complex-1 and cytoplasmic sequestration of Forkhead box protein O1 (Foxo1). Multiple lines of evidence indicate that sustained activity of Akt and mTOR drives T cells toward terminal differentiation. For instance, increased activation of Akt by interleukin-12 (IL-12),17 expression of a constitutively active form of Akt,18 and deletion of Foxo119 have all been shown to promote the formation of KLRG1+ effector T cells. Therefore, because constitutively active Akt induces terminal differentiation, we hypothesized that pharmacologic inhibition of the Akt pathway could inhibit the differentiation of CD8+ T cells. In this study, we demonstrate that by inhibiting the Akt pathway, we can generate highly potent MiHA-specific CD8+ T cells ex vivo. These Akt-inhibited CD8+ T cells showed superior expansion potential upon removal of the Akt inhibitor, which results in a superior antitumor effect in mice. By using hematopoietic-restricted MiHAs, this strategy is expected to be a safe and potent additive T-cell therapy after allo-SCT.
Materials and methods
Patient and donor material
Peripheral blood (PB) mononuclear cells were isolated by using Ficoll-Hypaque gradient (GE Healthcare, Madison, WI) from buffy coats of healthy donors, selected for HLA-A2 or -B7 positivity (Sanquin Blood Supply Foundation, Nijmegen, The Netherlands) or from PB samples from patients at several time points after allo-SCT. Allo-SCT patients were treated as described previously.20 Briefly, patients underwent HLA-matched allo-SCT for a hematologic malignancy after nonmyeloablative conditioning regimens. After conditioning, patients received a partial T-cell–depleted graft, with 0.5 ± 0.3 × 106 CD3+ T cells per kilogram (mean ± standard deviation). GVHD prophylaxis consisted of cyclosporine A only. Patients and healthy donors had given their written informed consent, which was approved by the Radboud university medical center Institutional Review Board.
In vitro Akt inhibitor VIII treatment and priming of MiHA-specific T cells
CD8+ cells were selected by using CD8 microbeads (via magnetic-activated cell sorting [MACS]; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s protocol and were resuspended in RPMI (Gibco Invitrogen) medium supplemented with 10% fetal calf serum (FCS; Integro, The Netherlands) or human serum (HS; PAA Laboratories). Next, cells were stimulated with (PD-L 1/2 silenced) DCs, generated as described previously.21,22 These were loaded with 5 µM MiHA peptide (HA1, VLHDDLLEA23 ; ARHGDIB, LPRACWREA24 ) in medium without serum for 30 minutes at 37°C and were added to the culture with a DC:T-cell ratio of 1:10 to 1:20. Cell cultures were supplemented with 50 U/mL IL-2 (Chiron, Emeryville, CA), 5 ng/mL IL-7 (ImmunoTools), and 5 ng/mL IL-15 (R&D Systems, Minneapolis, MN). When indicated, 8 µM Akt inhibitor VIII (50% inhibitory concentration, 58 nM, 210 nM, and 2.12 µM for Akt1, Akt2, and Akt3, respectively; Merck Millipore, Darmstadt, Germany) or dimethylsulfoxide was added as control. Medium, including cytokines and Akt inhibitor, was refreshed every 2 to 3 days, and MiHA-specific T-cell cultures were restimulated once per week with DCs and analyzed by flow cytometry.
Flow cytometry
To analyze surface markers, cells were resuspended in phosphate-buffered saline plus 0.5% bovine serum albumin, stained with 0.2 μg of phycoerythrin and allophycocyanin–labeled tetramers containing the MiHA-specific peptide for HA1.HLA-A2 (ARHGDIB.HLA-B7), HY.HLA-A2 (FIDSYICQV), HY.HLA-B7 (SPSVDKARAEL), CMV.HLA-A2 (NLVPMVATV), or CMV.HLA-B7 (RPHERNGFTVL), and incubated for 15 minutes in the dark at room temperature (RT). For additional markers, cells were labeled with the following antibodies: CD3 (UCHT1; BioLegend, San Diego, CA), CD8 (3B5; Invitrogen, Carlsbad, CA), CD45RO (UCHL1; Beckman Coulter, Brea, CA), CCR7 (G04H7; BioLegend), CD27 (O323; eBioscience), CD28 (IM2071U; Beckman Coulter), CD45RA (H100; BioLegend), CD62L (DREG56; BioLegend), CD95 (4B2; Beckman Coulter), CD127 (A019D5; BioLegend), and CXCR4 (12G5; BioLegend). Labeling was performed at 4°C in the dark for 30 minutes and measured on a Cyan-ADP nine-color analyzer (Beckman Coulter), FACS ARIA, or Gallios flow cytometer (Beckman Coulter). Analysis was performed by using Summit (Dako) or Kaluza (Beckman Coulter) software.
RNA extraction and real-time PCR quantification
RNA of fluorescence-activated cell sorter–purified MiHA-specific CD8+ T cells was extracted by using Quick-RNA Microprep kit (Zymo Research) according to the manufacturer’s instructions. Complementary DNA was synthesized by using M-MLV-reverse transcriptase (Invitrogen) in a standard reaction after which real-time polymerase chain reaction (PCR) was performed by using the following TaqMan gene expression assays (Applied Biosystems): Bcl6 (Hs00153368), EOMES (Hs00172872), FOXO1 (Hs01054576), GZMB (Hs01554355), ID2 (Hs04187239), ID3 (Hs00954037), IFNG (Hs00989291), KLRG1 (Hs00929964), PRDM1 (Hs00153357), PRF1 (Hs99999108), TBX21 (Hs00203436), and TCF7 (Hs00175273). Expression levels were calculated relative to glyceraldehyde-3-phosphate dehydrogenase (4326317E-1010038; Applied Biosystems) by using the ΔΔCt method and expressed relative to control cultures.
Functionality assays
After 14 days of priming, cells were cultured in Iscove modified Dulbecco medium (IMDM) + 10% HS supplemented with 50 U/mL IL-2, 5 ng/mL IL-7, and 5 ng/mL IL-15 and stimulated with MiHA-loaded (5 µM) irradiated 174CEM.T2 (T2) cells25 in a T2:T-cell ratio of 1:10. Medium and cytokines were refreshed every 2 to 3 days. On day 7, flow cytometry analysis was performed to determine the number of MiHA-specific CD8+ T cells. To determine functionality, rechallenged cells were stimulated overnight with MiHA peptide (5 µM) in the presence of anti-CD107a (H4A3; BioLegend), and Brefeldin A was added after 1 hour (1 ng/µL; BD Biosciences). The next day, cells were stained for extracellular surface markers and resuspended in 4% Paraformaldehyde for 10 minutes at RT. Then, cells were incubated in 0.1% saponin (Sigma) buffer containing 10% FCS for 10 minutes at RT, followed by intracellular staining for anti-CD137 (41BB; BioLegend) and anti-interferon gamma (IFN-γ) (B27; BD Biosciences) for 30 minutes at 4°C, after which cells were fixed in 1% Paraformaldehyde and measured on the Gallios flow cytometer.
Flow cytometry–based cytotoxicity assays were performed by using carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled (1.5 µM) tumor and Epstein-Barr virus (EBV) cell lines, in which 18 000 target cells were cocultured with 3000 MiHA-specific T cells in IMDM plus 10% HS. U266, UM6, UM9, and patient or donor EBV-transduced lymphoblastoid cell lines were cultured in IMDM plus 10% FCS. UM6 cultures were supplemented with 22.5 ng/mL IL-6 (ImmunoTools). Target cells alone were used as controls. After overnight incubation at 37°C, cells were harvested, and viable target cells were quantified by flow cytometry. Killing percentage was calculated as follows: 100 – (absolute No. of viable CFSE+ target cells cocultured with T cells/absolute No. of viable CFSE+ target cells cultured without T cells) × 100.
In vivo evaluation of proliferative self-renewal capacity and antitumor effect of MiHA-specific CD8+ T cells
NOD/SCID/IL2Rγnull (NSG) mice, originally purchased from The Jackson Laboratories, were housed and bred in the Radboud university medical center Central Animal Laboratory. Female mice from 6 to 20 weeks of age were used. All animal experiments were approved by the Animal Experimental Committee of the Radboud university medical center and were conducted in accordance with institutional and national guidelines (permit No. 10300). The tumor model was created by injecting 105 luciferase-expressing UM9 multiple myeloma cells (UM9.Luc) into the right femur of mice by insertion of a 25-gauge Hamilton needle through the knee joint. Tumor cell growth was monitored once per week by bioluminescence imaging as described previously.26 Signals >3 × 104 were considered specific. Mice with high tumor load on day 7 as a result of misinjection of the needle (so that tumor cells were not injected into the bone) and outliers were excluded to create homogeneous groups. Mice were injected intravenously with (MACS-enriched) primed CD8+ T cells containing equal numbers of MiHA-specific CD8+ T cells, and CD4+ T cells were co-infused to support engraftment. Then, mice were vaccinated intraperitoneally once per week with 0.5 × 106 autologous PD-L silenced DCs loaded with MiHA peptide. To support survival of MiHA-specific T cells, 0.5 μg recombinant human IL-15 (ie, 2500 U; Miltenyi) was administered intraperitoneally every 2 to 3 days for 3 weeks. PB and spleens were analyzed by tetramer staining using flow cytometry. Mice were euthanized when the palpable tumor reached 1 cm in diameter or when one of the following criteria was met: severe weight loss, poor coat and skin condition, static activity, or paraplegia.
Statistical analysis
The data were analyzed by using Graphpad Prism 4.0. Statistical significance was analyzed by using a (paired) t test and one- or two-way analysis of variance followed by a Bonferroni post hoc test as indicated in the figure legends. P < .05 was considered significant.
Results
Low frequency of circulating Tcm and Tscm cells in allo-SCT patients
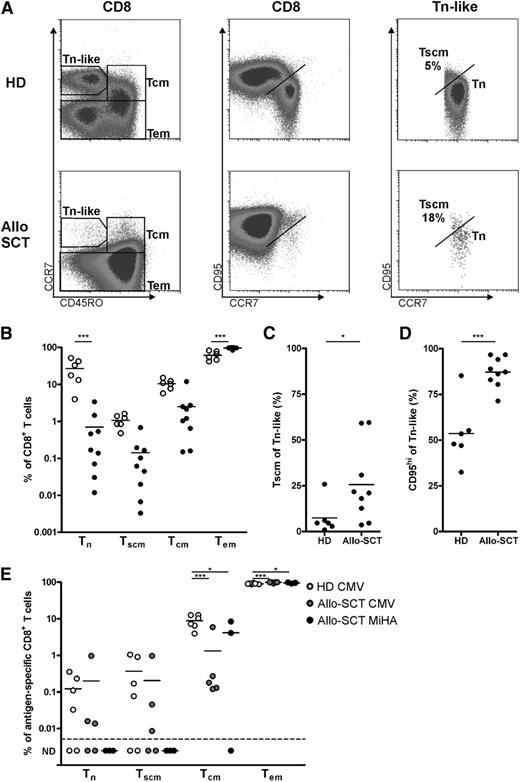
Human memory T cells with stem cell–like characteristics have been identified among antigen-experienced CD8+ T cells in healthy donors. To investigate the existence of Tscm cells relatively early after allo-SCT, we analyzed PB mononuclear cells of allo-SCT patients and healthy donors by flow cytometry. CD8+ T-cell subsets were divided into naive T cells (Tn), Tscm, Tcm, and Tem cells on the basis of expression of CD45RO, CCR7, CD27, and CD95 (Figure 1A and supplemental Figure 1 available on the Blood Web site). As we have shown previously,27,28 most CD8+ T cells displayed the Tem phenotype after allo-SCT (Table 1 and Figure 1B). Whereas in healthy controls, 27.0% ± 18.4% of the CD8+ T cells were Tn cells, after allo-SCT, CD8+ T cells contained less than 0.7% ± 1.1% Tn cells. Similarly, Tscm levels accounted for not more than 0.1% ± 0.2% in allo-SCT patients compared with 1.1% ± 0.4% within the CD8+ T-cell population of healthy controls. This occurred despite the observation that in allo-SCT patients, the population of Tscm cells was increased within the Tn-like population compared with healthy controls (Figure 1C), as shown previously by Cieri et al.29 This was also observed when Tscm cells were defined as CD95hi within Tn-like cells, resulting in >85% Tscm cells within Tn-like CD8+ T cells after allo-SCT (Figure 1D).
Low CD8+ Tscm in patients after allo-SCT. CD8+ T cells of (○) healthy donors (HD; n = 6) and (●) allo-SCT patients (n = 8 to 9) were analyzed for their differentiation status by flow cytometry. (A) Representative plot showing subset discrimination. (B) Summary of memory subsets within CD8+ T cells. (C) Tscm cells within Tn-like cells gated as shown in (A) or as (D) CD95hi in HD and allo-SCT patients. (E) Memory subsets within antigen-specific T cells in healthy donor (CMV, n = 6) and allo-SCT patients (CMV, n = 5; MiHA, n = 3). ND, not detectable. Statistical analysis was performed by using (B,E) a 2-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test or (C-D) a one-tailed Student t test. *P < .05; ***P < .001.
Low CD8+ Tscm in patients after allo-SCT. CD8+ T cells of (○) healthy donors (HD; n = 6) and (●) allo-SCT patients (n = 8 to 9) were analyzed for their differentiation status by flow cytometry. (A) Representative plot showing subset discrimination. (B) Summary of memory subsets within CD8+ T cells. (C) Tscm cells within Tn-like cells gated as shown in (A) or as (D) CD95hi in HD and allo-SCT patients. (E) Memory subsets within antigen-specific T cells in healthy donor (CMV, n = 6) and allo-SCT patients (CMV, n = 5; MiHA, n = 3). ND, not detectable. Statistical analysis was performed by using (B,E) a 2-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test or (C-D) a one-tailed Student t test. *P < .05; ***P < .001.
Patient characteristics
| Patient . | Disease . | Sex . | Age at allo-SCT (years) . | Conditioning regimen . | CMV status R/D* . | Sample (days)† . | CD8+ T cells . | Antigen-specific T cells . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tn (%) . | Tscm (%) . | Tcm (%) . | Tem (%) . | Antigen . | Frequency (%)‡ . | Tn (%) . | Tscm (%) . | Tcm (%) . | Tem (%) . | |||||||
| 1 | NHL | F | 48 | Cy + Ida + TBI | NA | 264 | 0.14 | 0.06 | 2.72 | 97.08 | — | — | — | — | — | — |
| 2 | CML | M | 47 | Cy + Bus | +/+ | 172 | 1.45 | 0.19 | 0.65 | 97.70 | CMV.HLA-B7 | 4.86 | 0.01 | 0.01 | 0.13 | 99.85 |
| 3 | AML | M | 50 | Cy + Ida + TBI | +/+ | 68 | 0.01 | <0.01 | 0.16 | 99.82 | CMV.HLA-B7 | 27.51 | ND | ND | 0.18 | 99.82 |
| 4 | MM | M | 59 | Cy + Flu | –/+ | 63 | 0.03 | 0.01 | 0.15 | 99.81 | CMV.HLA-B7 | 10.69 | ND | ND | 0.12 | 99.88 |
| 5 | AML | M | 50 | Cy + Ida + TBI | +/– | 205 | 0.46 | 0.68 | 1.20 | 97.65 | CMV.HLA-B7 | 9.91 | 0.02 | 0.05 | 0.27 | 99.67 |
| 6 | AML | M | 40 | Cy + TBI | +/– | 79 | 0.07 | 0.10 | 2.50 | 97.33 | CMV.HLA-A2 | 0.28 | 0.97 | 0.97 | 5.91 | 92.16 |
| 7 | AML | M | 60 | Cy + Ida + TBI | NA | 90 | 0.17 | 0.04 | 1.04 | 98.75 | HY.HLA-B7 | 0.50 | ND | ND | 4.09 | 95.91 |
| 8 | AML | M | 26 | Cy + Ida + TBI | NA | 104 | 0.54 | 0.02 | 2.15 | 97.30 | HY.HLA-A2 | 0.45 | ND | ND | ND | 100.00 |
| 9 | NHL | M | 28 | Cy + Ida + TBI | NA | 196 | 3.40 | 0.16 | 11.99 | 84.45 | HY.HLA-A2 | 0.19 | ND | ND | 8.41 | 91.59 |
| Patient . | Disease . | Sex . | Age at allo-SCT (years) . | Conditioning regimen . | CMV status R/D* . | Sample (days)† . | CD8+ T cells . | Antigen-specific T cells . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tn (%) . | Tscm (%) . | Tcm (%) . | Tem (%) . | Antigen . | Frequency (%)‡ . | Tn (%) . | Tscm (%) . | Tcm (%) . | Tem (%) . | |||||||
| 1 | NHL | F | 48 | Cy + Ida + TBI | NA | 264 | 0.14 | 0.06 | 2.72 | 97.08 | — | — | — | — | — | — |
| 2 | CML | M | 47 | Cy + Bus | +/+ | 172 | 1.45 | 0.19 | 0.65 | 97.70 | CMV.HLA-B7 | 4.86 | 0.01 | 0.01 | 0.13 | 99.85 |
| 3 | AML | M | 50 | Cy + Ida + TBI | +/+ | 68 | 0.01 | <0.01 | 0.16 | 99.82 | CMV.HLA-B7 | 27.51 | ND | ND | 0.18 | 99.82 |
| 4 | MM | M | 59 | Cy + Flu | –/+ | 63 | 0.03 | 0.01 | 0.15 | 99.81 | CMV.HLA-B7 | 10.69 | ND | ND | 0.12 | 99.88 |
| 5 | AML | M | 50 | Cy + Ida + TBI | +/– | 205 | 0.46 | 0.68 | 1.20 | 97.65 | CMV.HLA-B7 | 9.91 | 0.02 | 0.05 | 0.27 | 99.67 |
| 6 | AML | M | 40 | Cy + TBI | +/– | 79 | 0.07 | 0.10 | 2.50 | 97.33 | CMV.HLA-A2 | 0.28 | 0.97 | 0.97 | 5.91 | 92.16 |
| 7 | AML | M | 60 | Cy + Ida + TBI | NA | 90 | 0.17 | 0.04 | 1.04 | 98.75 | HY.HLA-B7 | 0.50 | ND | ND | 4.09 | 95.91 |
| 8 | AML | M | 26 | Cy + Ida + TBI | NA | 104 | 0.54 | 0.02 | 2.15 | 97.30 | HY.HLA-A2 | 0.45 | ND | ND | ND | 100.00 |
| 9 | NHL | M | 28 | Cy + Ida + TBI | NA | 196 | 3.40 | 0.16 | 11.99 | 84.45 | HY.HLA-A2 | 0.19 | ND | ND | 8.41 | 91.59 |
AML, acute myeloid leukemia; Bus, busulphan; CML, chronic myeloid leukemia; Cy, cyclophosphamide; D, donor; F, female; Ida, idarubicin; M, male; MM, multiple myeloma; NA, not applicable; ND, not detectable; NHL, non-Hodgkin lymphoma; R, recipient; TBI, total body irradiation.
Cytomegalovirus status of recipient and donor prior to allo-SCT. All 5 studied patients had no active CMV infection at time of sampling after allo-SCT.
Sample, time of sample collection in days after allo-SCT
Frequency, percentage of tetramer+ cells within CD8+ T cell population.
Antigen-experienced CD8+ T cells mostly display Tcm and Tem phenotypes, although Tscm cells have been described as being present within this population.14,29,30 We determined the composition of cytomegalovirus (CMV)- and MiHA-specific CD8+ T cells as described above. Similar to total CD8+ T cells, MiHA-specific and CMV-specific CD8+ T cells of allo-SCT patients contained a higher frequency of Tem cells compared with CMV-specific T cells from healthy donors (Figure 1E). Furthermore, CMV- and MiHA-specific T cells in allo-SCT patients contained lower levels of Tcm cells compared with healthy donors. Although Tscm cells could be clearly detected in CMV-specific CD8+ T cells of healthy controls and some allo-SCT patients, the MiHA-specific T-cell population did not contain detectable levels of Tscm. Altogether, these data emphasize the need to apply adjuvant approaches to increase the frequency of long-lived, tumor-reactive stem cell–like T cells posttransplant.
Generation of stem cell–like MiHA-specific CD8+T cells by inhibiting Akt signaling
Because constitutively active Akt induces terminal T-cell differentiation, we studied whether pharmacologic inhibition of the Akt pathway could inhibit ex vivo differentiation of CD8+ T cells and favor the generation of stem cell–like T cells. To set up culture conditions for optimal expansion of stem cell–like T cells, we first stimulated CD8+ Tn cells with anti-CD3/CD28 beads in the presence of combinations of the cytokines IL-2, IL-7, and IL-15 shown to be involved in memory and Tscm generation.29,31-33 Although variation was seen between cultures, no clear effect on T-cell differentiation was observed with any of the cytokine combinations (supplemental Figure 2). Next, stimulations were performed in the presence or absence of Akt inhibitor VIII, which selectively inhibits Akt1/Akt2 activity. We observed a delay in proliferation (supplemental Figure 3), but most importantly, Akt-inhibited CD8+ T cells showed reduced differentiation as manifested by higher expression of CD45RA and CCR7 compared with control cultures (supplemental Figure 3).
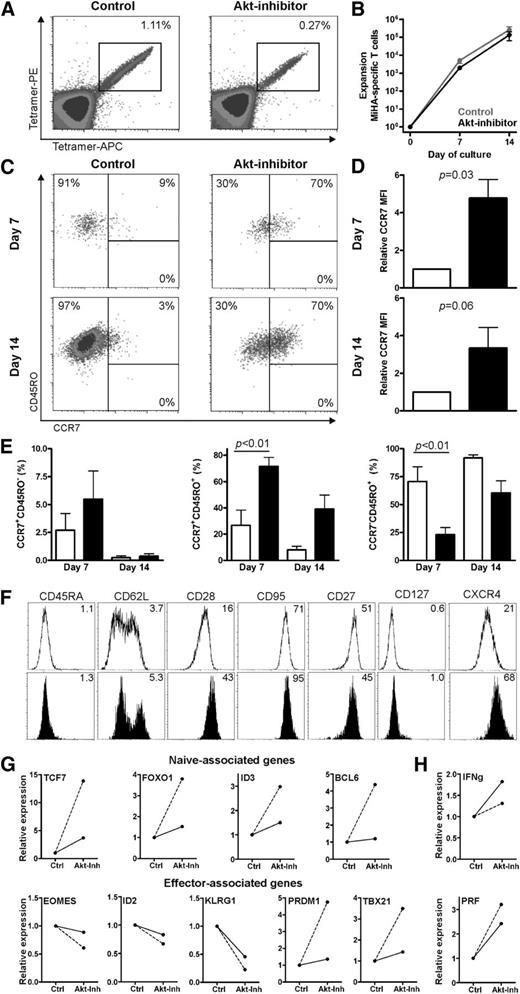
Next, we investigated whether MiHA-specific CD8+ T cells with an early differentiated phenotype could be generated by using the Akt inhibitor. Therefore, we stimulated purified CD8+ T cells from a MiHA-negative donor (containing naive MiHA-specific CD8+ T cells precursors) with MiHA-peptide loaded autologous DCs in the presence or absence of the Akt inhibitor. On days 7 and 14 after stimulation, the presence of MiHA-specific CD8+ T cells and their differentiation phenotypes were analyzed. Although the frequency of expanded MiHA-tetramer+ CD8+ T cells between the Akt-inhibited and noninhibited cultures varied in the different experiments (n = 4; [mean ± standard error of the mean] control [0.30 ± 0.53] vs Akt-inhibited cultures [0.14 ± 0.10] on day 14) (Figure 2A), the overall numbers of MiHA-specific CD8+ T cells on days 7 and 14 were comparable (Figure 2B). As expected for control conditions, the vast majority of MiHA-specific CD8+ T cells expressed CD45RO with no detectable levels of CCR7 after 2 weeks of culture (Figure 2C,E). Interestingly, inhibition of the Akt pathway significantly inhibited T-cell differentiation, because these T cells dominantly displayed a Tcm-like CCR7+CD45RO+ phenotype (P < .01; Figure 2C,E). Although an average ± standard error of 72% ± 7% of Akt-inhibited MiHA-specific CD8+ T cells on day 7 of culture had a CCR7+CD45RO+ phenotype, this was 27% ± 12% in the culture without Akt inhibitor (P < .01). After restimulation with peptide-loaded DCs and culturing for up to 14 days, 39% ± 11% of expanded Akt-inhibited MiHA-specific CD8+ T cells vs only 8% ± 3% without Akt inhibitor displayed the CCR7+CD45RO+ phenotype. This was also reflected in the median fluorescence intensity of CCR7 in Akt-inhibited MiHA-specific CD8+ T cells, which was higher when compared with control cultures (P = .03 on day 7 and P = .06 on day 14; Figure 2D).
Inhibiting Akt signaling during priming reserved T-cell differentiation of MiHA-specific CD8+ T cells. CD8+ T cells were cultured with peptide-loaded DCs for 7 days with or without 8 µM Akt inhibitor (inh). Medium-containing cytokines and Akt inhibitor were refreshed every 2 to 3 days. Flow cytometry analysis was performed on days 7 and 14 to determine T-cell differentiation of the MiHA-specific CD8+ T cells. (A) Representative tetramer staining on day 14 of culture. (B) Expansion of MiHA-specific T cells, calculated from an estimated precursor frequency of 1:107 (n = 4). (C) Representative staining for T-cell differentiation based on CCR7 and CD45RO expression, gated on MiHA-specific CD8+ T cells. (D) Median fluorescence intensity (MFI) of CCR7 expression of MiHA-specific CD8+ T cells relative to control (open bars, control; solid bars, Akt-inhibitor; n = 4). (E) Differentiation determined as in (C) of MiHA-specific CD8+ T cells on days 7 and 14 of culture (open bars, control; solid bars, Akt-inhibitor; n = 4). (F) Additional phenotypical analysis of MiHA-specific CD8+ T cells on day 14 of culture in the absence (open) or presence (solid) of 8 µM Akt inhibitor. (G-H) MiHA-specific CD8+ T cells cultured for 14 days in the absence or presence of 8 µM Akt inhibitor were fluorescence-activated cell sorter (FACS) purified and then analyzed by PCR. Two cultures of independent donors are shown; dashed line: 3% vs 60% and solid line: 20% vs 41% CCR7+CD45RO+ cells in control and Akt-inhibited MiHA-specific CD8+ T cells, respectively. Statistical analysis was performed by using (E) a 2-way ANOVA followed by a Bonferroni post hoc test or (D) paired Student t test. APC, allophycocyanin; PE, phycoerythrin.
Inhibiting Akt signaling during priming reserved T-cell differentiation of MiHA-specific CD8+ T cells. CD8+ T cells were cultured with peptide-loaded DCs for 7 days with or without 8 µM Akt inhibitor (inh). Medium-containing cytokines and Akt inhibitor were refreshed every 2 to 3 days. Flow cytometry analysis was performed on days 7 and 14 to determine T-cell differentiation of the MiHA-specific CD8+ T cells. (A) Representative tetramer staining on day 14 of culture. (B) Expansion of MiHA-specific T cells, calculated from an estimated precursor frequency of 1:107 (n = 4). (C) Representative staining for T-cell differentiation based on CCR7 and CD45RO expression, gated on MiHA-specific CD8+ T cells. (D) Median fluorescence intensity (MFI) of CCR7 expression of MiHA-specific CD8+ T cells relative to control (open bars, control; solid bars, Akt-inhibitor; n = 4). (E) Differentiation determined as in (C) of MiHA-specific CD8+ T cells on days 7 and 14 of culture (open bars, control; solid bars, Akt-inhibitor; n = 4). (F) Additional phenotypical analysis of MiHA-specific CD8+ T cells on day 14 of culture in the absence (open) or presence (solid) of 8 µM Akt inhibitor. (G-H) MiHA-specific CD8+ T cells cultured for 14 days in the absence or presence of 8 µM Akt inhibitor were fluorescence-activated cell sorter (FACS) purified and then analyzed by PCR. Two cultures of independent donors are shown; dashed line: 3% vs 60% and solid line: 20% vs 41% CCR7+CD45RO+ cells in control and Akt-inhibited MiHA-specific CD8+ T cells, respectively. Statistical analysis was performed by using (E) a 2-way ANOVA followed by a Bonferroni post hoc test or (D) paired Student t test. APC, allophycocyanin; PE, phycoerythrin.
To further analyze the level of T-cell differentiation of the Akt-inhibited cells, additional phenotypical and gene analyses were performed. Interestingly, we observed that CD62L and CD28, two surface markers expressed by less differentiated T cells, were expressed on Akt-inhibited MiHA-specific CD8+ T cells to a higher level compared with controls (Figure 2F). Furthermore, CD95, IL-7 receptor alpha (IL-7Rα, CD127), and CXCR4, which are highly expressed on stem cell–like CD8+ T cells, were expressed in larger amounts on Akt-inhibited cells. To determine T-cell differentiation at the transcriptional level, control and Akt-inhibited MiHA-specific CD8+ T cells were fluorescence-activated cell sorter sorted, and gene expression analysis was performed by quantitative PCR. Consistent with the phenotypic data, our data showed that genes encoding several transcriptional regulators involved in the generation and maintenance of long-lived memory cells, including TCF7, FOXO1, ID3, and BCL614,34 had greater expression in Akt-inhibited compared with control MiHA-specific CD8+ T cells (Figure 2G). Conversely, EOMES, ID2, and KLRG1 transcriptional regulators implicated in effector T-cell differentiation and senescence14,35 were expressed at lower levels upon inhibition of the Akt pathway. Interestingly, comparing ex vivo–expanded Akt-inhibited CD8+ T cells with naturally occurring T-cell subsets, the expression of TCF7 and KLRG1 was found to be between that of Tn and Tcm cells (supplemental Figure 4). Surprisingly, the effector-associated transcription factors PRDM1 and TBX21, which encode Blimp1 and T-bet respectively,35 were expressed in larger amounts in Akt-inhibited CD8+ T cells. Because all these genes are connected, it is possible that feedback loops are responsible for this observation.34 Consistent with the greater expression of PRDM1 and TBX21, Akt-inhibited MiHA-specific CD8+ T cells had increased messenger RNA expression of the effector molecules IFNg and PRF1 (Figure 2H) in our study. Combined, these data show that inhibition of Akt signaling reserves T-cell differentiation of ex vivo generated MiHA-specific CD8+ T cells but simultaneously favors the establishment of some effector gene features.
Akt-inhibited MiHA-specific CD8+ T cells have superior expansion capacity
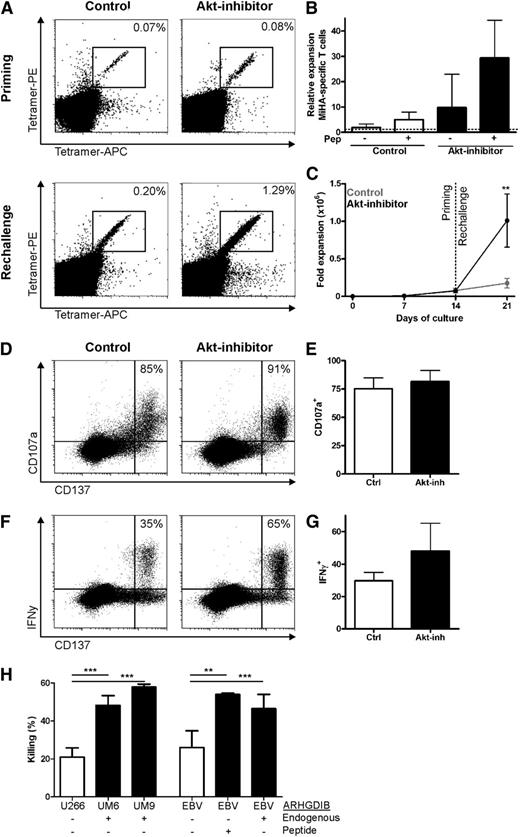
To determine whether the presence of effector traits had detrimental impact on the stemness of Akt-inhibited MiHA-specific CD8+ T cells, we evaluated their proliferative capacity upon restimulation after withdrawal of the Akt inhibitor. Therefore, primed CD8+ T cells were restimulated with T2 cells loaded with the MiHA peptide. This antigen re-encounter resulted in profound expansion of MiHA-specific CD8+ T cells, whereas stimulation with nonloaded T2 cells induced minimal proliferation (Figure 3A-B). Notably, MiHA-specific CD8+ T cells primed in the presence of the Akt inhibitor showed significantly more expansion compared with control (29-fold vs fivefold expansion; range, 8- to 59- vs 3- to 8-fold; Figure 3A-B). Importantly, this resulted in a profound increase in the number of MiHA-specific CD8+ T cells from Akt-inhibited cells after rechallenge (P < .01; Figure 3C), indicating that the high levels of PRDM1 were insufficient to instruct a program of replicative senescence in Akt-inhibited cells.
Enhanced expansion capacity of functional Akt-inhibited MiHA-specific CD8+ T cells in vitro. MiHA-specific CD8+ T cells cultured for 14 days in the absence or presence of 8 µM Akt inhibitor were rechallenged with peptide-loaded T2 cells and cultured for 7 days without Akt inhibitor. Medium with cytokines was refreshed every 2 to 3 days. Flow cytometry analysis was performed on day 7 to determine the number of MiHA-specific T-cells. (A) Representative tetramer staining on day 14 of priming and after 7 days of rechallenge. (B) Relative expansion to input of MiHA-specific CD8+ T cells during rechallenge of 3 independent donors, mean + standard error of the mean (SEM). (C) Expansion of MiHA-specific CD8+ T cells, calculated from an estimated precursor frequency of 1:107, during priming and rechallenge of 3 independent donors, mean + SEM. (D-G) Rechallenged cells were stimulated overnight with 5 µM peptide and stained for CD107a, IFN-γ, and CD137. Representative plots of CD137 and (D) CD107a or (F) IFN-γ staining within CD8+ T cells. Percentages represent CD107a+ or IFN-γ+ cells within CD137hi cells. Percentage of (E) CD107a+ or (G) IFN-γ+ within CD137hi cells was analyzed for 2 independent cultures, mean + SEM. Statistical analysis was performed by using a 2-way ANOVA followed by a Bonferroni post hoc test. (H) Killing of CFSE-labeled target cell lines with or without endogenous ARHGDIB expression or peptide loading, by MACS-enriched ARHGDIB-specific T cells (45% ARHGDIB-specific CD8+ T cells) derived from a rechallenged Akt-inhibited culture (n = 3). **P < .01; ***P < .001.
Enhanced expansion capacity of functional Akt-inhibited MiHA-specific CD8+ T cells in vitro. MiHA-specific CD8+ T cells cultured for 14 days in the absence or presence of 8 µM Akt inhibitor were rechallenged with peptide-loaded T2 cells and cultured for 7 days without Akt inhibitor. Medium with cytokines was refreshed every 2 to 3 days. Flow cytometry analysis was performed on day 7 to determine the number of MiHA-specific T-cells. (A) Representative tetramer staining on day 14 of priming and after 7 days of rechallenge. (B) Relative expansion to input of MiHA-specific CD8+ T cells during rechallenge of 3 independent donors, mean + standard error of the mean (SEM). (C) Expansion of MiHA-specific CD8+ T cells, calculated from an estimated precursor frequency of 1:107, during priming and rechallenge of 3 independent donors, mean + SEM. (D-G) Rechallenged cells were stimulated overnight with 5 µM peptide and stained for CD107a, IFN-γ, and CD137. Representative plots of CD137 and (D) CD107a or (F) IFN-γ staining within CD8+ T cells. Percentages represent CD107a+ or IFN-γ+ cells within CD137hi cells. Percentage of (E) CD107a+ or (G) IFN-γ+ within CD137hi cells was analyzed for 2 independent cultures, mean + SEM. Statistical analysis was performed by using a 2-way ANOVA followed by a Bonferroni post hoc test. (H) Killing of CFSE-labeled target cell lines with or without endogenous ARHGDIB expression or peptide loading, by MACS-enriched ARHGDIB-specific T cells (45% ARHGDIB-specific CD8+ T cells) derived from a rechallenged Akt-inhibited culture (n = 3). **P < .01; ***P < .001.
Next, we sought to determine whether the greater expression of TBX21 and effector molecules in Akt-inhibited MiHA-specific CD8+ T cells would confer functional advantages to these cells after antigen restimulation. Degranulation by CD107a was clearly observed for the CD137hi cells, which was equal for both MiHA-specific CD8+ T cells of control cultures and those primed in the presence of the Akt inhibitor (Figure 3D-E). In addition, for both cultures, IFN-γ production was observed within the CD137hi cells upon peptide stimulation (Figure 3F-G). Interestingly, a trend toward higher IFN-γ production was observed for MiHA-specific CD8+ T cells primed in the presence of the Akt inhibitor compared with controls, suggesting that higher TBX21 and IFNγ messenger RNA levels after priming in the presence of the Akt inhibitor might provide functional benefits. Subsequently, we tested whether the rechallenged Akt-inhibited MiHA-specific T cells could also recognize endogenously processed antigen on target cells. Therefore, MACS-enriched ARHGDIB-specific T cells, which were rechallenged from Akt-inhibited T cells, were cocultured with various target cells. This showed that the multiple myeloma cell lines UM6 and UM9, which endogenously express the MiHA ARHGDIB, were highly susceptible to killing (Figure 3H). In addition, killing of EBV-transformed cell lines was observed upon endogenous antigen or after loading ARHGDIB peptide. These findings demonstrate that priming of MiHA-specific CD8+ T cells in the presence of the Akt inhibitor resulted in unique CCR7+CD45RO+ stem cell–like T cells with superior proliferation capacity and high functional activity upon in vitro re-exposure.
Akt-inhibited MiHA-specific CD8+ T cells show enhanced expansion potential in vivo
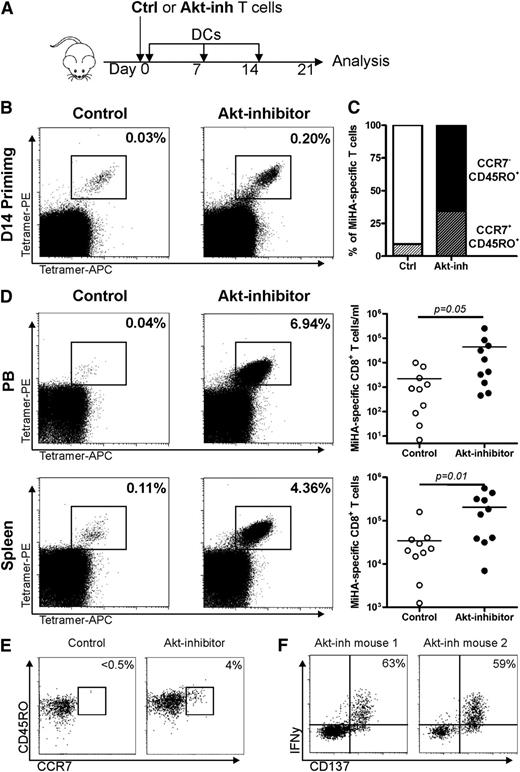
To test the in vivo expansion potential of Akt-inhibited MiHA-specific CD8+ T cells, ex vivo–generated CD8+ T cells containing equal numbers of MiHA-specific T cells were injected into NSG mice (Figure 4A). At the time of adoptive transfer, MiHA-specific CD8+ T cells of the control culture were >90% CCR7–CD45RO+, although 35% of the Akt-inhibited MiHA-specific CD8+ T cells still displayed a stem cell–like CCR7+CD45RO+ phenotype (Figure 4B-C). Autologous CD4+ T cells were co-infused to support engraftment, and peptide-loaded DC vaccination was given once per week to boost the activation and outgrowth of MiHA-specific CD8+ T cells. After 3 weeks, mice were euthanized, and spleen and PB were analyzed for the presence of MiHA-specific CD8+ T cells. Tetramer+ cells could be detected in PB and spleen of mice injected with either control or Akt-inhibited cultured cells (Figure 4D). Consistent with our in vitro observations, we found more robust MiHA-specific CD8+ T-cell expansion in PB and spleen of mice injected with Akt-inhibited cells (P = .05 and P = .01, respectively; Figure 4D). Notably, in PB and spleen alone, we had already observed up to 8 and 37 times more MiHA-specific CD8+ T cells than the infused product vs only 0.4 and 7 times in PB and spleen of control mice. These findings clearly demonstrate the superior in vivo expansion potential of the Akt-inhibited MiHA-specific CD8+ T cells.
Akt-inhibited MiHA-specific CD8+ T cells show enhanced expansion capacity in immunodeficient mice. (A) CD8+ T cells containing equal amounts (5500) of MiHA-specific T cells, cultured for 14 days in the absence or presence of 8 µM Akt inhibitor, were infused in NSG mice. Equal numbers of CD4+ T cells (3 × 106) were co-infused to support engraftment, resulting in a CD4:MiHA-specific T-cell ratio of 1:490. Mice were vaccinated once per week with DCs and supported with IL-15 every 2 to 3 days. On day 21, mice were euthanized and PB and spleen were analyzed by flow cytometry for the presence of MiHA-specific CD8+ T cells. (B) Tetramer staining and (C) differentiation consistency of cultured cells prior to infusion. (D) Representative tetramer staining and absolute numbers of MiHA-specific CD8+ T cells in PB and spleen (n = 10 mice per group). (E) T-cell differentiation phenotype of control and Akt-inhibited MiHA-specific CD8+ T cells after in vivo rechallenge; of each group, 1 representative mouse of 3 is shown. (F) IFN-γ production of Akt-inhibited MiHA-specific CD8+ T cells after in vivo rechallenge and overnight peptide stimulation; 2 independent mice are shown. Percentages represent IFN-γ+ cells within CD137hi cells. Statistical analysis was performed by using a one-tailed Student t test.
Akt-inhibited MiHA-specific CD8+ T cells show enhanced expansion capacity in immunodeficient mice. (A) CD8+ T cells containing equal amounts (5500) of MiHA-specific T cells, cultured for 14 days in the absence or presence of 8 µM Akt inhibitor, were infused in NSG mice. Equal numbers of CD4+ T cells (3 × 106) were co-infused to support engraftment, resulting in a CD4:MiHA-specific T-cell ratio of 1:490. Mice were vaccinated once per week with DCs and supported with IL-15 every 2 to 3 days. On day 21, mice were euthanized and PB and spleen were analyzed by flow cytometry for the presence of MiHA-specific CD8+ T cells. (B) Tetramer staining and (C) differentiation consistency of cultured cells prior to infusion. (D) Representative tetramer staining and absolute numbers of MiHA-specific CD8+ T cells in PB and spleen (n = 10 mice per group). (E) T-cell differentiation phenotype of control and Akt-inhibited MiHA-specific CD8+ T cells after in vivo rechallenge; of each group, 1 representative mouse of 3 is shown. (F) IFN-γ production of Akt-inhibited MiHA-specific CD8+ T cells after in vivo rechallenge and overnight peptide stimulation; 2 independent mice are shown. Percentages represent IFN-γ+ cells within CD137hi cells. Statistical analysis was performed by using a one-tailed Student t test.
To investigate the self-renewal potential of the Akt-inhibited MiHA-specific CD8+ T cells, we analyzed the phenotype of the cells after in vivo rechallenge. For both cultures, most in vivo expanded MiHA-specific CD8+ T cells displayed the CCR7–CD45RO+ phenotype. However, within the in vivo expanded Akt-inhibited MiHA-specific CD8+ T cells obtained from spleen, there was still a clearly detectable population that expressed both CCR7 and CD45RO (Figure 4E). Interestingly, the absolute number of CCR7+CD45RO+ MiHA-specific CD8+ T cells per spleen was more pronounced following adoptive transfer of Akt-inhibited CD8+ T cells (average ± standard deviation: 332 ± 92 in control vs 4066 ± 2587 in mice infused with Akt-inhibited MiHA-specific CD8+ T cells (P = .03). Finally, we evaluated the functionality of the in vivo expanded MiHA-specific CD8+ T cell in vaccinated mice. In spleens of mice injected with noninhibited T cells, no clear CD137+ or IFN-γ+ T cells could be detected (data not shown), but clear CD137 upregulation was observed within the splenic cell population of mice infused with Akt-inhibited MiHA-specific CD8+ T cells (Figure 4F). Within these CD137+-activated CD8+ T cells, ∼60% produced IFN-γ, reflecting the high functionality of these cells. Collectively, in this immunodeficient mouse model, we demonstrated that Akt-inhibited MiHA-specific CD8+ T cells exhibit a superior proliferative potential, possible self-renewal capacity, and high functionality upon antigen re-encounter.
Superior antitumor effect by Akt-inhibited MiHA-specific T cells
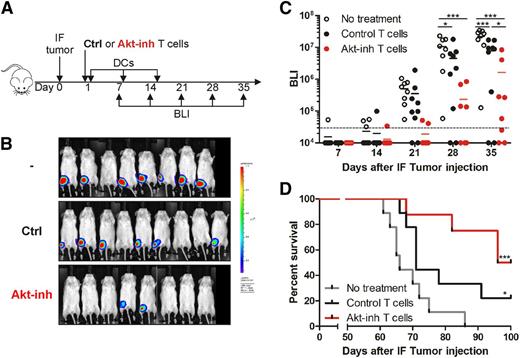
To test the antitumor potency of our Akt-inhibited MiHA-specific T cells, mice were injected intrafemurally with UM9.Luc multiple myeloma cells, which endogenously express the HLA-B7–restricted MiHA ARHGDIB (Figure 5A). The next day, mice were treated with no cells, control ARHGDIB-specific CD8+ T cells, or Akt-inhibited ARHGDIB-specific CD8+ T cells. To effectively boost the antitumor response of the adoptively transferred ARHGDIB-specific CD8+ T cells, mice were vaccinated once per week with DCs, thus providing effective antigenic stimulation in the presence of appropriate costimulation. Tumor growth in bone marrow was monitored over time via bioluminescence imaging. Nontreated mice displayed growth of the tumor, resulting in a detectable tumor in all mice on day 21 and further progression on days 28 and 35 (Figure 5B-C). Interestingly, on day 28, 2 of 9 mice treated with control MiHA-specific CD8+ T cells were still tumor free, and the average tumor load was significantly lower compared with nontreated mice (P < .05). But more strikingly, mice treated with Akt-inhibited MiHA-specific CD8+T cells showed clear delay in tumor growth, with 4 of 8 mice tumor free on day 28. Their tumor load was significantly lower compared with nontreated (P < .001) as well as control treated (P < .05) mice on days 28 and 35. Moreover, mice were observed for survival, in which nontreated mice died on day 60 and onward, with a median survival of 66 days (Figure 5D). Mice treated with control T cells had a prolonged median survival up to 71 days (P < .05). Interestingly, mice treated with Akt-inhibited T cells survived significantly longer, with a median survival of 98 days, and 4 of 8 mice were still alive at the end of the experiment (day 100; P < .001). These findings indicate the superior antitumor effect of Akt-inhibited MiHA-specific CD8+ T cells.
Superior antitumor effect of Akt-inhibited MiHA-specific CD8+ T cells. (A) NSG mice received an intrafemural (IF) injection with UM9 multiple myeloma cells expressing luciferase. On day 1, mice were treated with 50 000 MACS-enriched MiHA-specific CD8+ T cells after culture with (17% CCR7+CD45RO+; n = 8) or without Akt inhibitor (12% CCR7+CD45RO+; n = 9). To support engraftment, CD4+ T cells (0.3 × 106) were co-infused, resulting in a CD4:MiHA-specific T-cell ratio of 1:6. Mice were vaccinated once per week with DCs and received IL-15 every 2 to 3 days. Nontreated mice (n = 8) received only IL-15. From day 7 to day 35, bioluminescence imaging (BLI) was performed once per week to determine the tumor load, after which mice were monitored for survival. (B) BLI images on day 35 and (C) quantified signal corrected for background of the tumor-injected femur over time. Statistical analysis was performed by using a 2-way ANOVA followed by a Bonferroni post hoc test. (D) Survival of mice was analyzed according to a Mantel-Cox test; significance shown compared with no treatment. *P < .05; ***P < .001.
Superior antitumor effect of Akt-inhibited MiHA-specific CD8+ T cells. (A) NSG mice received an intrafemural (IF) injection with UM9 multiple myeloma cells expressing luciferase. On day 1, mice were treated with 50 000 MACS-enriched MiHA-specific CD8+ T cells after culture with (17% CCR7+CD45RO+; n = 8) or without Akt inhibitor (12% CCR7+CD45RO+; n = 9). To support engraftment, CD4+ T cells (0.3 × 106) were co-infused, resulting in a CD4:MiHA-specific T-cell ratio of 1:6. Mice were vaccinated once per week with DCs and received IL-15 every 2 to 3 days. Nontreated mice (n = 8) received only IL-15. From day 7 to day 35, bioluminescence imaging (BLI) was performed once per week to determine the tumor load, after which mice were monitored for survival. (B) BLI images on day 35 and (C) quantified signal corrected for background of the tumor-injected femur over time. Statistical analysis was performed by using a 2-way ANOVA followed by a Bonferroni post hoc test. (D) Survival of mice was analyzed according to a Mantel-Cox test; significance shown compared with no treatment. *P < .05; ***P < .001.
Discussion
Donor-derived CD8+ T cells are the main contributors to an effective GVT effect after allo-SCT. Therefore, it is highly important that these CD8+ T cells are effective in creating a robust memory response that results in long-lasting antitumor immunity. As in our previously published studies27,28 and shown in this study, circulating CD8+ T cells in cancer patients after allo-SCT display mainly a terminally differentiated Tem phenotype, with only low levels of CD8+ Tn cells and early differentiated memory subsets present. Although we could verify the increase in the highly functional Tscm population within Tn-like cells as described by Cieri et al,29 these CD8+ Tscm cells contributed only minimally to the total CD8+ T-cell population because of the low Tn-like cell numbers. The potentially suboptimal memory pool was even more noticeable within antigen-experienced CD8+ T cells because MiHA-specific CD8+ T cells after allo-SCT contained undetectable levels of Tscm cells. Therefore, we speculated that this may result in a short-lived antitumor reaction, which can be improved by applying additional posttransplant adoptive MiHA-based CD8+ T-cell therapy.
Because relapse remains a major problem after allo-SCT,36 development of adjuvant immunotherapies is essential. One of the current treatment options consists of nonselected donor lymphocyte infusion, of which only a relatively low number of naive precursors is targeting hematopoietic-restricted MiHAs to mount selective GVT responses. Therefore, development of adoptive T-cell therapy based on the transfer of enriched MiHA-specific CD8+ T cells targeting hematopoietic-restricted antigens could greatly enhance GVT responses in the absence of GVHD.37 However, ex vivo expansion and culture of these MiHA-specific precursor cells using current protocols results in terminally differentiated effector T cells that probably show low antitumor potency.11,14 In this study, we aimed to generate MiHA-specific CD8+ T cells with limited T-cell differentiation.
Although we could confirm efficient inhibition of T-cell differentiation by mimicking Wnt-signaling, as was described by Gattinoni et al,12,14 the block of proliferation observed in these cultures resulted in inefficient priming and expansion of MiHA-specific CD8+ T cells (supplemental Figure 5). Various targets within the Akt pathway, such as inhibiting mTOR, could be potentially interesting for generating antigen-specific T cells with an early-memory phenotype. However, similar to TWS119, the concentration of rapamycin required for significant inhibition of T-cell differentiation might also dramatically inhibit T-cell growth, because it has been shown that rapamycin robustly inhibits T-cell proliferation.38,39 Conversely, we observed that inhibition of Akt signaling did not strongly interfere with expansion of MiHA-specific CD8+ T cells, which therefore could be used for clinical generation of additive tumor-specific T-cell therapy. The strategy for inhibiting Akt resulted in ex vivo expansion of CD8+ T cells displaying a minimally differentiated phenotype compared with control cultures without Akt inhibition in both phenotype and gene expression. Interestingly, the effector-associated genes PRDM1 and TBX21 were elevated in Akt-inhibited cells compared with controls. Feedback regulations have possibly exerted this effect, because multiple transcriptional factors, including Blimp1, T-bet, EOMES, TCF7, and ID2, have been shown to interact closely.34 In addition, our observed expression of TBX21 could also explain the higher IFN-γ in these cells, because a critical role of TBX21 has been described in the formation of IFN-γ–producing T cells.40 Although Akt-inhibited MiHA-specific CD8+ T cells appeared to have some increased effector traits, KLRG1 expression was much lower compared with noninhibited T cells and that found in the naturally occurring Tn and Tcm subsets.
Importantly, our Akt-inhibited CD8+ T cells were superior in proliferation, which resulted in highly functional effector cells. This superior proliferation capacity observed in Akt-inhibited T cells could be caused by a combination of less replicative senescence characteristics, greater expression of costimulatory molecules such as CD28, and increased expression of cytokine receptors such as IL-7Rα. For instance, it has been shown that T cells expressing higher levels of IL-7Rα survive and proliferate better than IL-7Rαlow T cells following antigen stimulation in human T cells and in mouse models.41,42 Importantly, possible self-renewal potential of Akt-inhibited CD8+ T cells was observed after 3 weeks of in vivo boosting with peptide-loaded DC vaccines. Although secondary adoptive transfer studies will give more insight into the true self-renewal potential of our T cells, our data suggest long-lived memory formation and restimulation potential of the Akt-inhibited MiHA-specific T cells.
Altogether, Akt inhibition of MiHA-specific CD8+ T cells resulted in a superior antitumor effect against the multiple myeloma cell line UM9. Because the intrafemoral injection in our human tumor xenograft mouse model resulted in tumor growth in the natural myeloma habitat, we showed here that our Akt-inhibited MiHA-specific CD8+ T cells are not only more functional in vivo but may also more efficiently home to the bone marrow via their increased expression of CXCR4.43,44
In conclusion, inhibiting Akt signaling during the ex vivo priming, expansion, and culture of MiHA-specific CD8+ T cells gives rise to potent tumor-reactive T cells. These Akt-inhibited MiHA-specific CD8+ T cells have stem cell–like properties resembling traits of Tcm and Tscm cells. Therefore, infusion of Akt-inhibited MiHA-specific CD8+ T cells in allo-SCT patients could mount a potent GVT reaction; especially when targeting hematopoietic-restricted MiHAs, a specific GVT effect without GVHD could be boosted. This could prevent relapse of hematologic tumors after allo-SCT. Furthermore, our strategy to inhibit Akt signaling during the generation of antigen-specific T cells could be broadly exploited for the generation of tumor antigen or virus-specific stem cell–like CD8+ T cells for adoptive immunotherapy in cancer and viral infections.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rob Woestenenk and the biotechnicians from the Radboud University Medical Center Central Animal Laboratory for their technical assistance and Marcel Ruiters from Synvolux Therapeutics, Anna Borodovsky from Alnylam, and Fons van der Loo for supplying research reagents.
Authorship
Contribution: A.B.v.d.W. designed and performed experiments and wrote the paper; N.M.P.v.d.W., F.M., and C.S.M.K. performed experiments; M.G.D.K. and J.H.F.F. provided materials; N.S., J.H.J., R.v.d.V., and L.G. provided advice and revised the paper; W.H. performed experiments, provided advice, and revised the paper; and H.D. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harry Dolstra, Department of Laboratory Medicine-Laboratory of Hematology, Radboud university medical center, Geert Grooteplein 8, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: harry.dolstra@radboudumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal