Abstract
Smoldering myeloma is a heterogeneous clinical entity where a subset of patients has an indolent course of disease that mimics monoclonal gammopathy of undermined significance, whereas others have a more aggressive course that has been described as “early myeloma.” It is defined as either serum M-protein ≥3 g/L or ≥10% monoclonal plasma cells in the bone marrow. There are currently no molecular factors to differentiate risks of progression for these patients. Current recommendations of therapy continue to be patient observation or patient enrollment in clinical trials. However, new definitions of active multiple myeloma recently agreed upon by the International Myeloma Working Group may alter the timing of therapy. On the basis of emerging data of therapy in these patients, it seems reasonable to believe that future recommendations for therapy of patients with smoldering myeloma will become an increasingly important topic. In this article, we review the current knowledge of this disease and risk factors associated with progression. We also examine biological insights and alterations that occur in the tumor clone and the surrounding bone marrow niche. Finally, we review clinical trials that have been performed in these patients and provide recommendations for follow-up of patients with this unique disease entity.
Introduction
Although the last decade has seen the development of effective targeted therapies for patients with multiple myeloma (MM), the clinical utility of targeted therapies has been hampered by the development of drug resistance, clonal evolution, and disease progression, making the quest for cure ever more elusive for MM.1,2 However, one may argue that the concept of initiating therapy at the time of symptomatic disease in MM is analogous to initiating therapy in patients with solid tumors only after the development of metastatic disease.3 Consequently, it may not be surprising that even with the best combinations of agents that are currently available, cure is not achieved for most patients with MM. Therefore, many have explored the question of whether the treatment of the precursor asymptomatic states of MM will lead to the ultimate prevention of progression and cure in MM.4
MM is consistently preceded by precursor states of monoclonal gammopathy of undermined significance (MGUS) and smoldering MM (SMM).4 These represent a continuum of progression of the tumor burden in the absence of symptoms or signs of end-organ damage. In this article, we review the current understanding of SMM including biological insights and clinically available risks of determining progression. Current recommendations of therapy continue to be patient observation or enrollment in clinical trials for most of these patients. However, new definitions of active MM and indications of therapy were recently agreed by the International Myeloma Working Group (IMWG). On the basis of the emerging data of therapy in SMM patients showing evidence of long and durable responses reflected in significantly improved progression-free survival (PFS) and overall survival (OS),5 it seems reasonable to believe that near-future recommendations for therapy of patients with SMM will become an increasingly important topic.
Case description
A 42-year-old pediatrician comes to the clinic for blood work that showed the presence of a monoclonal protein. She was in her usual state of health but developed recurrent sinus infections over the course of a year, and she attributed it to her exposure to sick children in the hospital. During her routine evaluations, her protein level was found to be elevated at 9.5 g/dL (range, 6.0-8.0 g/dL). Her physician ordered a serum protein electrophoresis that showed an immunoglobulin (Ig)A κ paraprotein of 3.5 g/dL. She indicates that she feels well and has no bone pain, fevers, or weight loss. Her blood counts reveal a normal complete blood count and differential and normal creatinine and calcium levels. Her κ light chains were elevated, and the ratio was 30. Her IgG and IgM levels were suppressed. She underwent further workup that revealed no lytic lesions on the skeletal survey, and her magnetic resonance imaging (MRI) showed no focal lesions. A bone marrow biopsy was performed that showed that 30% of the cellularity was comprised of plasma cells occurring singly and in small clusters and sheets. Flow cytometry demonstrated the presence of CD38, CD138, and CD56+ cells with excess cytoplasmic κ light chain staining in 95% of the plasma cells classified as abnormal plasma cells by immunophenotyping. Cytogenetics and fluorescence in situ hybridization revealed gain of chromosome 1q.
Understanding the molecular mechanisms of progression in SMM
To better understand the underlying risks of progression of this patient, we need to examine the underlying molecular alterations that occur in the tumor clone or in the surrounding bone marrow microenvironment that allow disease progression from early stages of MGUS to SMM to active symptomatic disease.
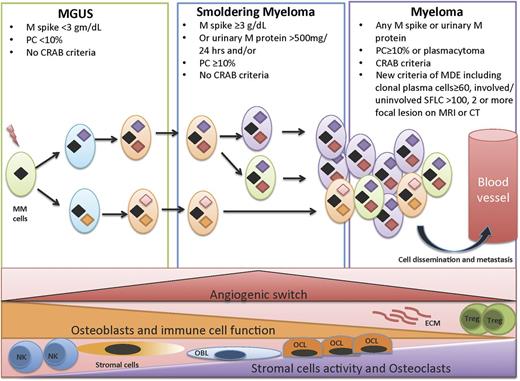
The “clonal evolution” model of cancer emerged amid ongoing advances in technology, especially in recent years. Next-generation sequencing has provided ever-higher resolution pictures of the genetic changes in cancer cells and heterogeneity in tumors where tumor progression proceeds in a branching rather than in a linear manner, leading to substantial clonal diversity and coexistence of wide genetic heterogeneity MM1,2,6,7 (Figure 1). Recent studies have shown intraclonal heterogeneity that occurs at different stages of progression in MM.8,9 A study10 of 4 patients with MGUS and another 3 patients with paired samples of SMM and overt MM from the same patients showed that both SMM and overt MM contain many subclones at low frequencies. The initiating events that lead to transformation of the plasma cells to malignant plasma cells is driven via the acquisition of a chromosomal translocation into the Ig loci or hyperdiploidy.4,11 These are considered founder genetic changes and are present in all clones. Secondary alterations then occur, which allow certain subclones to be fit for further progression and proliferation.
Clonal evolution in a permissive microenvironment. Progression from MGUS to SMM to symptomatic MM involves clonal evolution and heterogeneity, which is not only cell autonomous but also dependent on the interactions of the tumor cells with the bone marrow microenvironment. This includes immune cells such as T-regulatory cells (Tregs), myeloid derived suppressor cells (MDSCs), natural killer (NK) cells, osteoclasts, osteoblasts, angiogenesis, and MSCs.
Clonal evolution in a permissive microenvironment. Progression from MGUS to SMM to symptomatic MM involves clonal evolution and heterogeneity, which is not only cell autonomous but also dependent on the interactions of the tumor cells with the bone marrow microenvironment. This includes immune cells such as T-regulatory cells (Tregs), myeloid derived suppressor cells (MDSCs), natural killer (NK) cells, osteoclasts, osteoblasts, angiogenesis, and MSCs.
An international workshop assembled to review cytogenetic studies to evaluate whether MGUS and SMM cases have the same detectable anomalies that are often found in MM.12 Point mutations such as N-RAS, K-RAS, MYC upregulation,13 and gain or loss of chromosome 1q or 1p seem to correlate with disease progression from MGUS and SMM.14 Our patient indeed had gain of chromosome 1q, indicating that she may have more rapid progression.
A progressive increase in the incidence of copy number abnormalities from MGUS to SMM and to MM has been recently observed.15 Although MM has more copy number abnormalities than its precursor states, MGUS is as genetically aberrant as MM and does not appear to be associated with a particular chromosomal imbalance.15 Therefore, MM represents an expansion of altered clones that are already present in early precursor stages.15 Among the factors contributing to progression from MGUS to SMM to MM could be the average total number of point mutations, as it increments from MGUS to MM. Therefore, the transformation from MGUS/SMM to MM is likely to be an essential feature of clonal evolution and disease progression.10,16
The role of epigenetic regulators such as DNA methylation or histone modification is not well known in SMM. Aberrant promoter methylation has been described in MM.17-21 Specifically, p16 methylation represents one of the epigenetic aberrations that contribute to MM disease progression.22 Other studies of noncoding RNAs such as micro RNAs (miRNAs) have shown significant differences between MGUS and MM patients. In the vast majority of tumors, miRNAs are downregulated in clonal cells, thus suggesting their ability to act as tumor suppressors.23-26 Pichiorri et al27 profiled the expression of miRNAs from MGUS and MM patients and found miRNA-32 and the miRNA-17-92 cluster, including miRNA-19a and -19b, were significantly upregulated in MM and not in MGUS. A recent study showed that circulating miRNA could also be used for the prognosis of patients with MGUS and progression to MM.28
Although many factors regulating tumor growth are tumor cell autonomous, they are insufficient to induce dissemination and progression, and a permissive microenvironment is required for frank malignancy to emerge.29 A similar concept occurs in MM where the transition from MGUS to MM involves changes that occur due to the complex interaction of the malignant plasma cells with the microenvironment (Figure 1). These include upregulation of osteoblast receptor activator of nuclear factor kappa-B ligand (RANKL) expression and a decrease in osteoprotegerin, a decoy for RANKL that inhibits osteoclast differentiation.30,31 Interestingly, although lytic bone lesions are not seen in MGUS, the RANKL/osteoprotegerin ratio is higher in MGUS subjects, and they are at a higher risk of fractures than healthy controls.32 The interaction between MM cells and bone marrow stromal cells triggers nuclear factor-κB signaling pathway and interleukin-6 (IL-6) secretion by stromal cells (MSCs) and in turn vascular endothelial growth factor secretion by MM cells creating a paracrine loop that is optimal for MM growth.33 A recent study has shown the MSCs secrete specific exosomes that modulate the tumor clone, leading to rapid dissemination and tumor progression.34 In MM, increased angiogenesis of the bone marrow involves a complex interplay of proangiogenic and antiangiogenic molecules induced by plasma cells within the bone marrow microenvironment, with eventual balance tipped in favor of an “angiogenic switch,” as the disease transitions to MM from preceding MGUS and SMM.35 In a prospective clinical trial, microvessel density was low in samples of bone marrow obtained from patients with MGUS and increased in those with SMM and MM. In addition, several cytokines and growth factors have been implicated in myeloma pathogenesis and transition from MGUS to overt MM.36 Finally, an important step in the progression of tumors is evasion and suppression of the host immune system. There is an active immune response during the early stages of tumor growth in MGUS, which controls the growth but does not fully eliminate the tumor clone. As tumor growth progresses to the stages of SMM and active MM, there are associated cellular and humoral immune deficiencies,37 indicating that the evolution of disease in MM is associated with an immunosuppressive milieu that fosters immune escape.
How to diagnose patients with SMM and initial evaluation
SMM represents a clinically heterogeneous entity that has a higher risk of progression than MGUS, but in whom treatment is not indicated due to the lack of end-organ damage required for the diagnosis of MM.38 In 1978, Kyle et al coined the term MGUS, which was followed by the description of 6 patients described as having SMM by Kyle and Greipp in 1980.39 It was not until 2003 that the IMWG developed a consensus definition for MGUS and SMM, with MGUS being defined as the presence of serum M protein <3 g/dL with <10% monoclonal plasma cells in the bone marrow, whereas SMM was defined as either serum M protein ≥3 g/dL or ≥10% monoclonal plasma cells in the bone marrow40,41 (Table 1; Figure 2). These 2 entities had to show no evidence of end-organ damage, which was defined with the CRAB criteria of hypercalcemia (serum calcium ≥11.5 mg/dL), renal failure (defined by creatinine ≥1.95 with no other etiology), anemia (hemoglobin ≤10 g/dL or >2 g/dL below the lower limit of normal), or skeletal lesions (lytic lesions by skeletal survey, osteoporosis with pathologic fractures, or cord compression). Prior to the 2003 IMWG criteria,41 several other definitions of the same entity were introduced including indolent myeloma by Alexanian,42 evolving MM by Rosinol et al,43 and Durie Salomon stage I disease by Durie and Salmon.44
Definition of MGUS, SMM, and symptomatic MM
| MM classification . | International Myeloma Working Group criteria, 2010 version45 . |
|---|---|
| MGUS | Serum M protein <3 g/dL |
| Light-chain restricted bone marrow plasma cells <10% | |
| No end-organ damage | |
| SMM | Serum M protein >3 g/dL |
| Ad/or light-chain restricted bone marrow plasma cells >10% | |
| Or urinary monoclonal protein >500 mg per 24 hr | |
| No end-organ damage | |
| MM | Clonal bone marrow plasma cells ≥10% and/or biopsy-proven plasmacytoma |
| Presence of serum and/or urinary monoclonal protein at any level | |
| Evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder (CRAB criteria): hypercalcemia, serum calcium >0.25 mmol/L above upper limit of normal or >2.75 mmol/L (>1 mg/dL above upper limit of normal); renal insufficiency, serum creatinine >173 μmol/L (>2 mg/dL); anemia, normochromic, normocytic with a hemoglobin value of >2 g/dL below the lower limit of normal or a hemoglobin value <10 g/dL; bone lesions, lytic lesions or osteoporosis with compression fractures | |
| New definition of MM requiring therapy | Clonal bone marrow plasma cells ≥10% or biopsy proven plasmacytoma and |
| Any CRAB criteria as described above | |
| Or any myeloma defining events (MDE) as follows: clonal bone marrow plasma cell percentage* ≥60%47 ; an abnormal FLC ratio ≥100 (involved κ) or <0.01 (involved λ)48 ; and ≥2 focal lesions on MRI or PET-CT studies46,49 |
| MM classification . | International Myeloma Working Group criteria, 2010 version45 . |
|---|---|
| MGUS | Serum M protein <3 g/dL |
| Light-chain restricted bone marrow plasma cells <10% | |
| No end-organ damage | |
| SMM | Serum M protein >3 g/dL |
| Ad/or light-chain restricted bone marrow plasma cells >10% | |
| Or urinary monoclonal protein >500 mg per 24 hr | |
| No end-organ damage | |
| MM | Clonal bone marrow plasma cells ≥10% and/or biopsy-proven plasmacytoma |
| Presence of serum and/or urinary monoclonal protein at any level | |
| Evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder (CRAB criteria): hypercalcemia, serum calcium >0.25 mmol/L above upper limit of normal or >2.75 mmol/L (>1 mg/dL above upper limit of normal); renal insufficiency, serum creatinine >173 μmol/L (>2 mg/dL); anemia, normochromic, normocytic with a hemoglobin value of >2 g/dL below the lower limit of normal or a hemoglobin value <10 g/dL; bone lesions, lytic lesions or osteoporosis with compression fractures | |
| New definition of MM requiring therapy | Clonal bone marrow plasma cells ≥10% or biopsy proven plasmacytoma and |
| Any CRAB criteria as described above | |
| Or any myeloma defining events (MDE) as follows: clonal bone marrow plasma cell percentage* ≥60%47 ; an abnormal FLC ratio ≥100 (involved κ) or <0.01 (involved λ)48 ; and ≥2 focal lesions on MRI or PET-CT studies46,49 |
These studies are mandatory for risk stratification of patients with SMM and exclusion of patients with overt symptomatic MM or with the new classified patients with MDE.
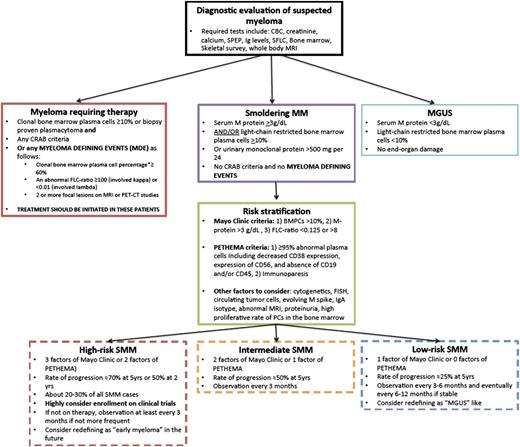
Proposed guidelines of follow-up and management of SMM. Patients suspected to have MM should first be defined as having MGUS, SMM, or myeloma requiring therapy. This includes the new classification of patients with MDE. For patients with SMM, these should then be stratified based on the Mayo Clinic criteria or PETHEMA criteria as having low-risk, intermediate-risk, or high-risk SMM. For high-risk SMM, we would highly recommend clinical trials or very close observation if not enrolled in a trial. We would consider redefining these patients in the future as early myeloma. For low-risk SMM, we would recommend less frequent monitoring if clinically stable and consider redefining these patients as MGUS-like. CRAB is defined as hypercalcemia (serum calcium ≥11.5 mg/dL), renal failure (defined by creatinine ≥1.95 with no other etiology), anemia (hemoglobin ≤10 g/dL or >2 g/dL below the lower limit of normal), or skeletal lesions (lytic lesions by skeletal survey, osteoporosis with pathologic fractures, or cord compression).
Proposed guidelines of follow-up and management of SMM. Patients suspected to have MM should first be defined as having MGUS, SMM, or myeloma requiring therapy. This includes the new classification of patients with MDE. For patients with SMM, these should then be stratified based on the Mayo Clinic criteria or PETHEMA criteria as having low-risk, intermediate-risk, or high-risk SMM. For high-risk SMM, we would highly recommend clinical trials or very close observation if not enrolled in a trial. We would consider redefining these patients in the future as early myeloma. For low-risk SMM, we would recommend less frequent monitoring if clinically stable and consider redefining these patients as MGUS-like. CRAB is defined as hypercalcemia (serum calcium ≥11.5 mg/dL), renal failure (defined by creatinine ≥1.95 with no other etiology), anemia (hemoglobin ≤10 g/dL or >2 g/dL below the lower limit of normal), or skeletal lesions (lytic lesions by skeletal survey, osteoporosis with pathologic fractures, or cord compression).
The studies required for staging patients with SMM are similar to those obtained for the diagnosis of MM and are shown in Table 2. SMM is diagnosed by the presence of a high enough tumor burden in the bone marrow and monoclonal protein but with the absence of symptoms or signs of end-organ damage that are characteristic of MM. Recent recommendations include MRI of the spine and pelvis or low-dose computed tomography (CT) scan that can predict for a more rapid progression to MM45,46 (see detailed section of imaging studies in SMM). Additional features have been recently added as criteria of clinical diagnosis of overt MM and initiation of therapy, which include bone marrow plasmacytosis ≥60%47 ; an abnormal free light chain (FLC) ratio of involved to uninvolved monoclonal light chain ≥10048 ; and/or ≥2 focal bone marrow lesions detected by functional imaging including positron emission tomography (PET)-CT and/or MRI.46,49
Diagnostic evaluation for SMM
| Exam, laboratory and imaging studies . | Studies to be performed . |
|---|---|
| Medical history and physical examination | Determine symptoms suggestive of symptomatic disease such as bone pain, weight loss, neuropathy, and rule out amyloidosis if any symptoms suggestive of AL amyloidosis |
| Blood and urine studies | Complete blood count and differential* |
| Chemistry profile including BUN, creatinine*, | |
| Total protein, LDH, calcium, phosphate | |
| β-2 microglobulin and albumin | |
| Serum protein electrophoresis, immunofixation,* | |
| Serum-free light chain analysis* | |
| Quantitative tests for IgG, IgA, and IgM | |
| 24-hour urine for UPEP and immunofixation | |
| NT-proBNP to rule out AL amyloidosis | |
| Bone marrow studies | Biopsy for histology* |
| Immunophenotype | |
| Cytogenetic analysis and fluorescence in situ hybridization focused on del(17p13), del(13q), del(1p12), ampl(1q21), t(11;14), t(4;14), and t(14;16) | |
| In the future, consider sequencing studies such as ClonoSIGHT, targeted DNA sequencing, or RNA sequencing if available | |
| Imaging | Skeletal survey* |
| Spine/pelvis MRI to rule lytic lesions* | |
| Optional PET/CT scan or low-dose CT scan to rule out lytic lesions |
| Exam, laboratory and imaging studies . | Studies to be performed . |
|---|---|
| Medical history and physical examination | Determine symptoms suggestive of symptomatic disease such as bone pain, weight loss, neuropathy, and rule out amyloidosis if any symptoms suggestive of AL amyloidosis |
| Blood and urine studies | Complete blood count and differential* |
| Chemistry profile including BUN, creatinine*, | |
| Total protein, LDH, calcium, phosphate | |
| β-2 microglobulin and albumin | |
| Serum protein electrophoresis, immunofixation,* | |
| Serum-free light chain analysis* | |
| Quantitative tests for IgG, IgA, and IgM | |
| 24-hour urine for UPEP and immunofixation | |
| NT-proBNP to rule out AL amyloidosis | |
| Bone marrow studies | Biopsy for histology* |
| Immunophenotype | |
| Cytogenetic analysis and fluorescence in situ hybridization focused on del(17p13), del(13q), del(1p12), ampl(1q21), t(11;14), t(4;14), and t(14;16) | |
| In the future, consider sequencing studies such as ClonoSIGHT, targeted DNA sequencing, or RNA sequencing if available | |
| Imaging | Skeletal survey* |
| Spine/pelvis MRI to rule lytic lesions* | |
| Optional PET/CT scan or low-dose CT scan to rule out lytic lesions |
These studies are mandatory for risk stratification of patients with SMM and exclusion of patients with overt symptomatic MM or with the new classified patients with MDE.
Imaging studies in SMM
One of the CRAB criteria for defining symptomatic MM is the presence of lytic lesions. This has been traditionally identified using radiological skeletal survey, which is still the gold standard for the initial workup of patients with MM.50 However, although this technique is safe and has minimal cost, it requires the loss of 30% to 50% of the bone mass before it detects lesions. MRI is able to assess the disease in the bone marrow itself independent from the growth pattern and therefore can provide information on the actual tumor burden.51 SMM patients with ≥2 focal bone marrow lesions were found to have a significantly shorter time to progression to active MM, making this one of the new criterion that will be used to initiate therapy for MM.46 In addition, CT scans can detect bone lesions at earlier time points and a low-dose CT scan is being used in some studies to determine end-organ damage. In addition to those, functional techniques such as PET (PET-CT or PET-MRI), dynamic contrast-enhanced MRI, and diffusion weighted imaging MRI allow imaging along with information regarding functional disease activity.49
Follow-up of SMM and evaluating risk factors and rate of progression
The incidence and prevalence of SMM in the population is not well defined. It has been estimated that it represents ∼8% to 20% of patients with MM.38 A recent review of the Swedish Myeloma Registry from 2008 to 2011 with a total of 2494 patients showed that 360 (14.4%) had SMM. Of the patients with SMM, 104 (28.8%) had high-risk disease (defined as an M protein level of ≥3 g/dL and plasma cell infiltration of ≥10%); these patients accounted for 4.2% of all patients with MM. On the basis of the world population as reference, the age-standardized incidence of SMM is 0.44 cases per 100 000 persons, and the incidence of high-risk disease is 0.14 cases per 100 000 persons.52
On the basis of a retrospective study from the Mayo Clinic, the overall risk of progression from SMM to MM was 10%/year for the first 5 years, 3%/year for the next 5 years, and 1%/year for the last 10 years, suggesting that the current definition of SMM is highly biologically and clinically heterogeneous.53
The follow-up of these patients and how often to monitor them for progression depends on their risk factors for progression as discussed in this section. The 2010 IMWG guidelines indicated that patients should be seen every 2 to 3 months for the first year, followed by every 4 to 6 months for 1 year, with eventual 6- to 12-month evaluations if clinically stable thereafter.45 The authors of this article would recommend closer follow-up for patients with high-risk SMM (Figure 2).
Indeed, SMM represents a heterogeneous clinical entity where a subset of patients have a very indolent course of disease that mimics an MGUS-like state, whereas others have a more aggressive course of disease that has been described as early myeloma or CRAB-negative myeloma. There are currently no molecular factors to differentiate these 2 clinically and biologically distinct entities of patients, and further studies are required to identify markers of progression of these patients.
The current factors associated with risk of progression are mainly based on the level of tumor burden in these patients assessed by the degree of tumor involvement in the bone marrow and the quantification of monoclonal protein in the peripheral blood. The 2 most widely used risk stratification methods are the Mayo clinic53,54 and the Programa para et Tratamiento de Hemopatias Malignas (PETHEMA) Spanish group classifications55 (Table 3). The Mayo Clinic criteria are primarily based on the levels of serum protein markers (serum protein electrophoresis and FLC assay) and the percent of bone marrow plasma cells in the bone marrow.53,54 The risk stratification of the PETHEMA Study Group focused on the use of multiparameter flow cytometry of the bone marrow to quantify the ratio of abnormal, neoplastic plasma cells to normal plasma cells and reduction of uninvolved Igs.55 Interestingly, a head-to-head comparison between the PETHEMA and the Mayo Clinic risk models showed significant discordance, reflected in many patients being high risk with one model and low risk with the other model.56
Risk stratification of SMM
| No. of risk factors . | Patients, n (%) . | Progression at 5 years . |
|---|---|---|
| Mayo Clinic criteria* | ||
| 1 | 76 (28) | 25% |
| 2 | 115 (42) | 51% |
| 3 | 82 (30) | 76% |
| PETHEMA criteria** | ||
| 0 | 28 (31) | 4% |
| 1 | 22 (25) | 46% |
| 2 | 39 (44) | 72% |
| No. of risk factors . | Patients, n (%) . | Progression at 5 years . |
|---|---|---|
| Mayo Clinic criteria* | ||
| 1 | 76 (28) | 25% |
| 2 | 115 (42) | 51% |
| 3 | 82 (30) | 76% |
| PETHEMA criteria** | ||
| 0 | 28 (31) | 4% |
| 1 | 22 (25) | 46% |
| 2 | 39 (44) | 72% |
N = 8955 . Risk factors: (1) ≥95% abnormal plasma cells, including decreased CD38 expression, expression of CD56, and absence of CD19 and/or CD45; and (2) immunoparesis.
Other risk factors that have been examined include the role of the IgA (vs IgG) isotype, the presence of proteinuria, circulating plasma cells, a high proliferative rate of bone marrow plasma cells, and abnormal MRI findings.4,38,57
Recent studies have reported that chromosomal abnormalities present in the plasma cells are also critical for the rate of progression in SMM. Two studies showed that that the presence of deletion 17p or t(4;14) is associated with the shortest time to progression (TTP) and that trisomies were a risk factor for progression from SMM to MM.11,58 Gains of 1q21 were also associated with increased risk for progression among patients with SMM.
Therefore, patients diagnosed with SMM should first be classified on the basis of progression risk factors (both by the Mayo Clinic criteria and by the PETHEMA criteria). In addition, other factors to be considered for high risk of progression should including cytogenetics, the number of circulating plasma cells, and the evolving nature of the M spike, as well as MRI findings. Of note, patients with the old classification of “ultra-high-risk” should now be reclassified as having overt MM that requires therapeutic intervention. These patients include those with bone marrow plasmacytosis ≥60%47 ; an abnormal FLC ratio ≥100 (involved κ) or <0.01 (involved λ)48 ; and/or ≥2 focal bone marrow lesions detected by functional imaging including PET-CT and/or MRI.46,49
Our patient was diagnosed with high-risk SMM by the Mayo Clinic and PETHEMA Criteria. Therefore, this patient may actually show more rapid progression of ∼70% to 80% at 5 years (see Figure 2 for our proposed guidelines of follow-up and management of patients with SMM).
Options of management in SMM: observation or early treatment
The current standard practice for patients diagnosed with SMM is to observe them without therapy as a watch-and-wait strategy.38,40 However, this paradigm may change as there is already a clinical trial showing a difference in PFS and OS in this patient population.5
Our patient showed significant progression after 1 year of follow-up, where a repeat bone marrow biopsy showed 70% involvement with plasma cells. The patient did not have any other criteria for symptomatic MM based on the CRAB criteria. However, based on the recent reclassification of some high-risk SMM patients as having overt myeloma with a “myeloma defining event,” our patient was started on therapy. Therefore, this recent change in the classification of these patients is critical as they fulfill the criteria of having a MDE and should be treated just like patients with symptomatic overt MM. These patients should be excluded from studies of SMM in all future clinical trials.
The hypothesis that early therapeutic intervention will lead to significant improvement in response has been examined for many years.38 There are 2 major ideas for therapeutic intervention: the first is prevention of progression and the second is definitive therapy to try and achieve complete remission with the hope that all subclones are eradicated at this early disease state and cure can be achieved.4
The major barrier to early intervention has been defining the group of patients who would truly benefit from this early treatment and would have otherwise shown progression to symptomatic disease. Indeed if SMM is a heterogeneous mix of patients with early myeloma and MGUS-like myeloma, then identification of those with early MM should allow for intervention only in those patients who truly warrant therapy. Unfortunately, there are no biological markers to define progressive disease in SMM other than the tumor burden markers that we discussed in the risk stratification section.
The first studies to examine the hypothesis of early intervention were conducted in the 1990s using melphalan and prednisone.59-62 These trials did not demonstrate a survival advantage, although they were not adequately powered to make definitive conclusions (Table 4). These were followed by studies using bisphosphonates in SMM (including 2 randomized controlled studies) that did not show improvement in OS or time to progression but did demonstrate fewer skeletal-related events.63 Thalidomide was the next agent to be tested in this patient population. It showed significant improvement in PFS in the thalidomide/zoledronic acid arm compared with the zoledronic acid alone arm (29 vs 14 months) but no difference in PFS as defined by CRAB events (49 vs 40 months; P < .18) or in OS (6-year OS, 70%).64-67
Select clinical trials in SMM
| Type of therapy . | Clinical trial design and outcome . | N of patients . | References . |
|---|---|---|---|
| Melphalan and prednisone (MP) | Retrospective cohort study of vincristine, adriamycin, and dexamethasone (VAD) vs MP. Because the treatment of MM remains palliative, chemotherapy should be withheld until symptoms | 23 SMM, 10 IMM | 60 |
| Initial vs delayed MP. Randomized-controlled trial. Similar response rate, response duration, and TTP of 12 months | 50 SMM and IMM (25/25) | 59 | |
| Initial vs delayed MP. TTP of about 12 months. No difference in OS (64 vs 71 months) | 145 DSSI | 61, 62 | |
| Observational study of delayed therapy: 54 DSS I. 2-year PFS 75%. Tumor specific OS 80% at 60 months | 54 DSSI | 71 | |
| Pamidronate or zolendronate | Single-arm, phase 2 trial; pamidronate vs observation. 5-year PFS 53% both arms. Skeletal-related events (SRE) 74% vs 39%, P = .009. Median OS 46 and 48 months | 177 SMM | 72,-74 |
| Open-label randomized controlled trial; zolendronate vs observation × 1 year. TTP not significant. SRE 55 vs 78%, P = .04. Zoledronate (ZLD) for 1 year decreased risk of skeletal-related disease, but TTP was similar (P = .83). OS no difference | 163 SMM | 63 | |
| Thalidomide | Single-arm, phase 2 trial phase 2 of thalidomide pamidronate. 4-year event-free survival (EFS) 60%. 4-year OS 91%. Median TTP 7 years; partial response (PR) identifies subset requiring earlier salvage therapy for symptomatic disease | 76 SMM | 64 |
| Single-arm pilot study of thalidomide. Median 35 months. OS 86 months. OS from treatment 49 months. Minimal response (MR) or better in 11/16. Microvessel density did not predict response | 19 SMM and I0 IMM | 66 | |
| Phase 2 of thalidomide. Patients were treated with thalidomide 100 to 200 mg. The response rate was 36% | 28 high risk SMM | 65 | |
| Phase 3 of thalidomide/ZLD vs ZLD | 68 SMM (35 to Thal/ZLD and 33 ZLD alone) | 67 | |
| Thalidomide +ZA vs ZA. 29 vs 14 months. 6-year OS >70% | 68 SMM | 67 | |
| IL-1 antagonist | Anakinra (IL-1 receptor antagonist). IL-1 antagonist +/− dexamethasone. Median PFS was 37.5 months. MR (n = 3), PR (n = 5). 8 patients stable on drug for 4 years | 47 SMM and IMM | 75 |
| Curcumin | Randomized, double-blind placebo-controlled crossover study. Administering 8 g dose of curcumin. Curcumin therapy decreased the free light-chain ratio, reduced the difference between clonal and monoclonal light-chain (dFLC) and involved free light-chain (iFLC) | 17 SMM | 76 |
| Lenalidomide and dexamethasone | Lenalidomide+dex vs observation. 2-year PFS 92% vs 30%, P < .001; 3-year OS 93% vs 76%, P < .04 | 119 high risk SMM | 5 |
| Type of therapy . | Clinical trial design and outcome . | N of patients . | References . |
|---|---|---|---|
| Melphalan and prednisone (MP) | Retrospective cohort study of vincristine, adriamycin, and dexamethasone (VAD) vs MP. Because the treatment of MM remains palliative, chemotherapy should be withheld until symptoms | 23 SMM, 10 IMM | 60 |
| Initial vs delayed MP. Randomized-controlled trial. Similar response rate, response duration, and TTP of 12 months | 50 SMM and IMM (25/25) | 59 | |
| Initial vs delayed MP. TTP of about 12 months. No difference in OS (64 vs 71 months) | 145 DSSI | 61, 62 | |
| Observational study of delayed therapy: 54 DSS I. 2-year PFS 75%. Tumor specific OS 80% at 60 months | 54 DSSI | 71 | |
| Pamidronate or zolendronate | Single-arm, phase 2 trial; pamidronate vs observation. 5-year PFS 53% both arms. Skeletal-related events (SRE) 74% vs 39%, P = .009. Median OS 46 and 48 months | 177 SMM | 72,-74 |
| Open-label randomized controlled trial; zolendronate vs observation × 1 year. TTP not significant. SRE 55 vs 78%, P = .04. Zoledronate (ZLD) for 1 year decreased risk of skeletal-related disease, but TTP was similar (P = .83). OS no difference | 163 SMM | 63 | |
| Thalidomide | Single-arm, phase 2 trial phase 2 of thalidomide pamidronate. 4-year event-free survival (EFS) 60%. 4-year OS 91%. Median TTP 7 years; partial response (PR) identifies subset requiring earlier salvage therapy for symptomatic disease | 76 SMM | 64 |
| Single-arm pilot study of thalidomide. Median 35 months. OS 86 months. OS from treatment 49 months. Minimal response (MR) or better in 11/16. Microvessel density did not predict response | 19 SMM and I0 IMM | 66 | |
| Phase 2 of thalidomide. Patients were treated with thalidomide 100 to 200 mg. The response rate was 36% | 28 high risk SMM | 65 | |
| Phase 3 of thalidomide/ZLD vs ZLD | 68 SMM (35 to Thal/ZLD and 33 ZLD alone) | 67 | |
| Thalidomide +ZA vs ZA. 29 vs 14 months. 6-year OS >70% | 68 SMM | 67 | |
| IL-1 antagonist | Anakinra (IL-1 receptor antagonist). IL-1 antagonist +/− dexamethasone. Median PFS was 37.5 months. MR (n = 3), PR (n = 5). 8 patients stable on drug for 4 years | 47 SMM and IMM | 75 |
| Curcumin | Randomized, double-blind placebo-controlled crossover study. Administering 8 g dose of curcumin. Curcumin therapy decreased the free light-chain ratio, reduced the difference between clonal and monoclonal light-chain (dFLC) and involved free light-chain (iFLC) | 17 SMM | 76 |
| Lenalidomide and dexamethasone | Lenalidomide+dex vs observation. 2-year PFS 92% vs 30%, P < .001; 3-year OS 93% vs 76%, P < .04 | 119 high risk SMM | 5 |
The most critical study of SMM that has reignited interest in therapeutic intervention in this patient population came from the PETHEMA group using lenalidomide and dexamethasone in comparison with observation. Mateos et al5 reported on 119 patients with high-risk SMM who received either observation or lenalidomide and dexamethasone in an open label randomized trial. Patients treated with lenalidomide and dexamethasone had a superior 3-year survival without progression to symptomatic disease (77% vs 30%; P < .001) and a superior 3-year OS (94% vs 80%; P = .03) from the time of registration. However, patients had to meet ≥1 of 2 sets of inclusion criteria based on a definition of high-risk disease, the Mayo Clinic criteria or the PETHEMA risk stratification criteria, with 40% of the patients in the trial included on the basis of flow cytometry criteria, which are not widely available, and the results were not stratified according to the definition of high-risk status. Therefore, there are some concerns regarding the generalizability of this study. This is important to note as we compare different clinical trials and the results obtained from these trials. Therefore, every effort should be made to collect information for all risk factors of progression when enrolling patients on these trials.
In addition, this study was criticized because of how asymptomatic biochemical progression was handled in both arms, the short OS of the abstention group, and the use of salvage therapy in the abstention group.52,68-70 Because of these concerns, further studies are needed before implementing therapeutic interventions as standard of care in patients with high-risk SMM. However, this trial was provocative enough and has triggered the development of many clinical trials that are ongoing to examine the role of therapy in this patient population (Table 5). Agents being tested include combinations of therapy to achieve deep responses such as carfilzomib/lenalidomide and dexamethasone, novel immunotherapies such as the Signaling Lymphocytic Activation Molecule family member 7 targeting agent elotuzumab, CD38 targeting antibodies, and programmed cell death-1 targeting antibodies among others.
Select ongoing clinical trials
| Type of therapy . | Clinical trial and design . | N of patients . | References . |
|---|---|---|---|
| Celoxicib vs placebo | Double-blind, randomized controlled trial aim: to test if celoxicib reduces the M protein concentration | 36 MGUS and SMM | Kalaycio 2004—ongoing |
| Lenalidomide vs observation | Open-label randomized controlled trial aim: to evaluate if lenalidomide extends TTP | 370* “High Risk” SMM | Lonial 2010—ongoing |
| Anti-KIR monoclonal antibody | Aim: to evaluate if anti-KIR reduces the M protein concentration >50% from baseline | 21 SMM | Landgren 2010—ongoing |
| BHQ880, a fully human, anti-Dickkopf1 (DKK1) neutralizing antibody | Single arm, open-label, phase 2 trial aim: to evaluate overall response rate | 58* “High Risk” SMM | Novartis Pharmaceuticals 2011—ongoing |
| Elotuzumab (humanized anti-CS1 monoclonal IgG1 antibody) | Aim: to assess the association between NK cell status and efficacy | 40* “High Risk” SMM | BMS Pharmaceuticals 2012—ongoing |
| Siltuximab (anti-IL-6 monoclonal antibody) | Randomized multicenter phase 2, blinded, placebo-controlled trial aim: 1-year PFS rate | 100* “High Risk” SMM | Janssen Pharmaceuticals 2012—ongoing |
| Carfilzomib, lenalidomide, and DEX | Single arm pilot (phase 2) trial aim: to evaluate overall response rate | 12 pilot + 18 expansion cohort (N=30) “High Risk” SMM | Landgren 2012—ongoing |
| Type of therapy . | Clinical trial and design . | N of patients . | References . |
|---|---|---|---|
| Celoxicib vs placebo | Double-blind, randomized controlled trial aim: to test if celoxicib reduces the M protein concentration | 36 MGUS and SMM | Kalaycio 2004—ongoing |
| Lenalidomide vs observation | Open-label randomized controlled trial aim: to evaluate if lenalidomide extends TTP | 370* “High Risk” SMM | Lonial 2010—ongoing |
| Anti-KIR monoclonal antibody | Aim: to evaluate if anti-KIR reduces the M protein concentration >50% from baseline | 21 SMM | Landgren 2010—ongoing |
| BHQ880, a fully human, anti-Dickkopf1 (DKK1) neutralizing antibody | Single arm, open-label, phase 2 trial aim: to evaluate overall response rate | 58* “High Risk” SMM | Novartis Pharmaceuticals 2011—ongoing |
| Elotuzumab (humanized anti-CS1 monoclonal IgG1 antibody) | Aim: to assess the association between NK cell status and efficacy | 40* “High Risk” SMM | BMS Pharmaceuticals 2012—ongoing |
| Siltuximab (anti-IL-6 monoclonal antibody) | Randomized multicenter phase 2, blinded, placebo-controlled trial aim: 1-year PFS rate | 100* “High Risk” SMM | Janssen Pharmaceuticals 2012—ongoing |
| Carfilzomib, lenalidomide, and DEX | Single arm pilot (phase 2) trial aim: to evaluate overall response rate | 12 pilot + 18 expansion cohort (N=30) “High Risk” SMM | Landgren 2012—ongoing |
Summary and recommendations
On the basis of the current definition, SMM is a not a unique biological entity but rather a step in the continuum of clonal evolution and progression of tumor plasma cells present in the bone marrow microenvironment that ultimately lead to symptomatic MM. However, the recognition of SMM provides a unique opportunity to understand the biological steps of progression in this fatal disease and to develop therapeutic interventions that can prevent/delay progression or even cure the disease by aggressively targeting the tumor cells before significant clonal heterogeneity occurs and before further immune dysfunction and microenvironmental dysregulation occurs. For many years, scientists have tried to test the hypothesis of early therapeutic intervention to prevent progression or cure myeloma but failed until the provocative results of the study using lenalidomide and dexamethasone showed that, indeed, early intervention can have a survival advantage. However, more studies are required before adopting these results in clinical practice. The major change in the current practice of SMM is that a subpopulation of high-risk SMM will now be reclassified as patients with overt MM who have MDE. These patients should be treated like those with symptomatic disease. For all other patients, the authors of this article suggest that patients with SMM should still be monitored carefully or enrolled on clinical trials to better assess the role of early intervention in this distinct group of patients. In the future, we believe that better molecular characterization of these patients can identify those with early myeloma who have a high risk of progression to symptomatic disease and should be treated vs those with MGUS-like stages who will not benefit from therapy. Although it remains to be formally tested and proven, one may speculate that early myeloma is genetically less adverse, and with optimal therapy, some patients could be cured with currently available drugs. Ongoing and future studies will hopefully provide answers to these important questions.
Acknowledgments
This work was supported by National Institutes of Health, National Cancer Institute grant R01CA 181683-01 and the Leukemia and Lymphoma Society.
Authorship
Contribution: I.M.G. and O.L. contributed to the writing of this review and opinion statements.
Conflict-of-interest disclosure: I.M.G. is on the advisory board of Celgene, Millennium, Onyx, Novartis, BMS, and Noxxon. O.L. has consulted and given scientific talks on behalf of Celgene, Millennium, Onyx, and Medscape.
Correspondence: Irene Ghobrial, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: Irene_ghobrial@dfci.harvard.edu; and Ola Landgren, Memorial Sloan Kettering Cancer Center, Myeloma Service, 1275 York Ave, New York, NY 10065; e-mail landgrec@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal