Key Points
Low doses of adoptively transferred donor CD4+ iNKT cells protect from GVHD while preserving graft-versus-tumor effects.
Donor CD4+ iNKT cells inhibit proliferation of alloreactive T cells and promote robust expansion of donor Tregs.
Abstract
Dysregulated donor T cells lead to destruction of host tissues resulting in graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT). We investigated the impact of highly purified (>95%) donor CD4+ invariant natural killer T (iNKT) cells on GVHD in a murine model of allogeneic HCT. We found that low doses of adoptively transferred donor CD4+ iNKT cells protect from GVHD morbidity and mortality through an expansion of donor CD4+CD25+FoxP3+ regulatory T cells (Tregs). These Tregs express high levels of the Ikaros transcription factor Helios and expand from the Treg pool of the donor graft. Furthermore, CD4+ iNKT cells preserve T-cell–mediated graft-versus-tumor effects. Our studies reveal new aspects of the cellular interplay between iNKT cells and Tregs in the context of tolerance induction after allogeneic HCT and set the stage for clinical translation.
Introduction
Dysregulated activation and proliferation of donor T cells following allogeneic hematopoietic cell transplantation (HCT) leads to immune-mediated destruction of host tissues resulting in graft-versus-host disease (GVHD).1 Most established therapeutic approaches involving immunosuppressive drugs to prevent or treat GVHD lead to a global suppression of T-cell function, have significant toxicities, and lead to increased risk of opportunistic infections. Adoptive transfer of donor CD4+CD25+FoxP3+ regulatory T cells (Tregs) has been studied in murine animal models, and promising results have been reported in haploidentical and umbilical cord blood HCTs.2-4 A deeper understanding of immune regulatory mechanisms holds promise for controlling dysregulated immune responses and improving outcomes after allogeneic HCT and for the treatment of other conditions, including severe autoimmune disorders, as well as for the induction of tolerance to transplanted organs.5
Despite their rarity in humans and mice, invariant natural killer T (iNKT) cells harbor potent immunomodulatory functions. They are characterized by rapid effector function upon stimulation of the semi-invariant T-cell receptor (TCRα Vα24-Jα18 in humans; Vα14-Jα18 in mice) with glycolipids.6,7 Host iNKT cells have an important tolerogenic impact on GVHD after reduced-intensity conditioning with total lymphoid irradiation and anti-thymocyte globulin (TLI/ATG).8 In this study, we investigated the impact of purified and adoptively transferred donor CD4+ iNKT cells on GVHD and graft-versus-tumor (GVT) effects in a murine model of allogeneic HCT.
Methods
Mice
Gender-matched female or male mice between 10 and 14 weeks of age were used for all experiments. BALB/c (H-2Kd), C57BL/6 (H-2Kb), and FVB (H-2Kq) mice were purchased from The Jackson Laboratory. C57BL/6 mice that expressed luciferase gene (luc+), Thy1.1, and CD45.1 were generated as described previously.9 C57BL/6 FoxP3 mutant mice expressing diphtheria toxin receptor (FoxP3DTR) were a kind gift from Dr Rudensky (Memorial Sloan Kettering Cancer Center, New York, NY) and were bred in our animal facility. Animal protocols were approved by the Institutional Animal Care and Use Committee of Stanford University.
Cell isolation
For the isolation of CD4+ iNKT cells, spleens were dispersed in phosphate-buffered saline (PBS; Life Technologies) with 2% fetal calf serum (FCS; Life Technologies) into single-cell suspensions, red blood cells were lysed with ammonium chloride buffer, and Fc receptors were blocked (Miltenyi Biotec) before B cells were depleted with CD45R (B220) MicroBeads (Miltenyi Biotec). iNKT cells were stained with PBS-57-CD1d tetramer phycoerythrin (PE; National Institutes of Health) and enriched with Anti-PE MicroBeads (Miltenyi Biotec). After magnetic-activated cell sorting, cells were stained for T-cell receptor β (TCR-β), CD4 and with live∕dead fixable dead cell stain (Life Technologies) and further purified on a FACSAria II cell sorter (BD Biosciences). To isolate donor Thy1.1+ Tregs from BALB/c recipient animals following HCT, single-cell suspensions from spleen and lymph nodes were enriched by magnetic-activated cell sorting (Miltenyi Biotec) for CD25+ T cells and sorted for live Thy1.1+CD4+CD25high T cells on a FACSAria II cell sorter. Donor CD4+ and CD8+ conventional T cells (Tcons) were prepared from splenocytes and lymph nodes and enriched with CD4 and CD8 MicroBeads (Miltenyi Biotec). T-cell–depleted bone marrow (TCD-BM) cells were prepared by flushing bones and depleting T cells with CD4 and CD8 MicroBeads (Miltenyi Biotec). Lymphocytes were isolated from GVHD target tissues for analysis. Livers were dispersed, and residual red blood cells were lysed with ammonium chloride buffer. Intrahepatic lymphocytes were isolated by density centrifugation with Percoll (GE Healthcare). Intestines were flushed with PBS and dissected into pieces. Intestinal lymphocytes were isolated by tissue digestion with 1 mg⋅mL−1 Collagenase IV (Life Technologies) for 30 minutes.
Depletion of Tregs from the graft
FoxP3DTR C57BL/6 mice were injected intraperitoneally with 50 µg⋅kg−1 diphtheria toxin (Sigma-Aldrich) dissolved in PBS on days −2 and −1 to deplete FoxP3-expressing cells. Control mice were injected with PBS only. On day 0, secondary lymphoid organs were harvested and processed to isolate Tcons as described previously. TCD-BM and CD4+ iNKT cells were both derived from untreated wild-type (WT) C57BL/6 mice.
Allogeneic bone marrow transplantation
BALB/c recipient mice were treated with lethal total body irradiation (TBI; 200 kV x-ray source; Kimtron) consisting of 2 doses of 4.0 Gy 4 hours apart. The tail vein was injected with 5.0 × 106 WT or CD45.1+ TCD-BM cells together with 1.0 × 106 WT or luc+/Thy1.1+ Tcons both from C57BL/6 mice on day 0. Donor CD4+ iNKT cells were co-injected on day 0 from C57BL/6 mice. Transplanted animals were housed in autoclaved cages with antibiotic water (sulfamethoxazole-trimethoprim; Hi-Tech Pharmacal). GVHD score was assessed as described previously.10 Briefly, weight, fur, skin, activity, and posture were evaluated, and a score of 0 to 2 was assigned to each group resulting in a maximal GVHD score of 10.
Tumor model
To investigate GVT activity, we used a B-cell lymphoma 1 (BCL1) and A20 lymphoma model expressing luciferase. On day −7 before HCT, 3000 luc+ BCL1 cells were intravenously injected into BALB/c recipients. Tumor engraftment was verified by bioluminescence imaging (BLI) before TBI. On day 0, 1.0 ×104luc+ A20 lymphoma cells were injected together with TCD-BM after TBI. After transplantation, tumor burden was assessed by BLI.
Histopathology
Tissues were fixed in 10% neutral buffered formalin. After 48 to 72 hours of formalin fixation, tissues were trimmed and processed routinely for microscopic examination after staining with hematoxylin and eosin. Stained tissue sections were evaluated for GVHD by a board-certified veterinary pathologist with an Olympus BX-41 microscope (Olympus). Representative digital photomicrographs were taken by using an Axioscope 2 Plus microscope (Carl Zeiss) with a Nikon DS-Ri1 digital microscope camera and NIS-Elements imaging software (Nikon).
Flow cytometric analysis
PBS-57-loaded and unloaded mCD1d tetramers were obtained from the National Institutes of Health Tetramer Facility. The following antibodies were purchased from BD Biosciences, eBioscience, or BioLegend: TCR-β (H57-597), CD4 (GK1.5), CD8 (53-6.7), B220 (RA3-6B2), CD11b (M1/70), Gr-1 (RB6-8C5), CD49b (DX5), Thy-1.1 (OX-7), CD45.1 (A20), CD45.2 (104), H-2Kb (AF6-88.5), CD25 (PC61), CD44 (IM7), FoxP3 (FJK-16s), Helios (22F6), TGF-β (LAP) (TW7-16B4), CTLA-4 (UC10-4B9), PD-1 (29F.1A12), Lag-3 (C9B7W), murine interferon γ (mIFN-γ; XMG1.2), and murine/human interleukin 5 (m/hIL-5; TRFK5). Isotype controls were purchased from the respective vendors. To stain dead cells, live∕dead fixable dead cell stain was used. Data were acquired on an LSR II flow cytometer (BD Biosciences), and analysis was performed with FlowJo 10.0.7 software (Tree Star).
CFSE-based cell proliferation assay
For analysis of cell proliferation, Thy1.1+ Tcons were resuspended in PBS and stained with CellTrace carboxyfluorescein diacetate succinimidyl ester (CFSE) cell proliferation kit (Life Technologies) for 5 minutes at 37°C. Immediately after staining, cells were washed 3 times in ice-cold RPMI 1640 (Mediatech) plus 10% FCS and finally resuspended in PBS. Lethally irradiated BALB/c mice were injected with 1.0 × 106 CFSE-labeled Thy1.1+ Tcons together with TCD-BM with or without CD4+ iNKT cells. The percentage of re-isolated proliferating Tcons was determined by flow cytometric analysis.
BLI
BLI was performed as described previously.11 Briefly, firefly luciferin (Biosynth) was injected intraperitoneally 10 minutes prior to image acquisition with an IVIS 29 or IVIS Spectrum imaging system (Xenogen). Images were analyzed with Living Image Software 4.2 (Xenogen).
Cytokine analysis
For intracellular cytokine staining, cells were stimulated with 20 ng⋅mL−1 phorbol myristate acetate (Sigma-Aldrich) and 1 µg⋅mL−1 ionomycin (Sigma-Aldrich) for 6 hours at 37°C and 5% CO2 in RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine (Mediatech), 100 U⋅mL−1 penicillin (Thermo Fisher Scientific), and 100 µg⋅mL−1 streptomycin (Thermo Fisher Scientific). Monensin (BD Biosciences) was used to block cellular protein transport. After staining surface antigens, cells were fixed and permeabilized (eBioscience) prior to staining of intracellular and intranuclear antigens. For quantitative measurement of serum cytokines, whole blood from recipient mice was obtained through puncture of the tail vein or heart after euthanasia. Serum was stored at −20°C until it was analyzed with a multiplex assay (Luminex).
Mixed lymphocyte reaction
Irradiated (30 Gy) BALB/c splenocytes (stimulators) were plated together with allogeneic Tcons (responders) at a 4:1 ratio and different doses of donor Thy1.1+ Tregs isolated on day +10 after HCT from mice treated with CD4+ iNKT cells. Following incubation for 96 hours, cells were pulsed with 1 µCi per well of thymidine (PerkinElmer), and thymidine incorporation was measured with a Wallac 1205 Betaplate counter (PerkinElmer).
Statistical analysis
Differences in animal survival (Kaplan-Meier survival curves) were analyzed with the log-rank test. All other comparisons were performed with the Student t test. P < .05 was considered statistically significant.
Results
Adoptively transferred CD4+ iNKT cells protect from GVHD in a dose-dependent manner
Upon stimulation, CD4+ iNKT cells produce cytokines characterized through a tolerogenic Th2 phenotype such as IL-4 and IL-13.12,13 Thus, we hypothesized that the adoptive transfer of CD4+ iNKT cells would improve GVHD morbidity and mortality. CD4+ iNKT cells were isolated from C57BL/6 donor spleens to a purity >95% (Figure 1A). Increasing doses of CD4+ iNKT cells were injected via tail vein together with 5.0 × 106 TCD-BM cells and 1.0 × 106 Tcons into BALB/c recipient mice after 8 Gy TBI on day 0. A significant survival benefit was observed for animals that received CD4+ iNKT cells at doses of 1.0 × 104 (P = .03) to 1.0 × 105 (P = .002) (Figure 1B). In addition, weight (Figure 1C) and GVHD scores (Figures 1D) improved with increasing numbers of CD4+ iNKT cells. Histopathology from GVHD target sites of BALB/c recipient mice obtained on days +10 and +35 after allogeneic HCT corroborated our clinical findings (Figure 1E). Furthermore, we found that adoptive transfer of CD4+ iNKT cells isolated from FVB donor mice also protected from lethal GVHD when 5.0 × 106 TCD-BM cells and 1.0 × 106 Tcons from FVB mice were transplanted into lethally irradiated BALB/c mice (P = .007) (supplemental Figure 1A-C available on the Blood Web site). Thus, highly purified and adoptively transferred donor CD4+ iNKT cells suppress Tcon-mediated alloimmunity in a dose-dependent manner in at least 2 different mouse strains.
Purified and adoptively transferred CD4+ iNKT cells protect from GVHD in a dose-dependent manner. (A) Magnetic-activated cell sorting (MACS) followed by fluorescence-activated cell sorting (FACS) of CD4+ iNKT cells. Shaded histogram curves depict CD4 isotype control. (B) Survival, (C) weight, and (D) GVHD score of BALB/c mice co-injected with 5.0 × 106 TCD-BM cells, 1.0 × 106 Tcons, and increasing doses of CD4+ iNKT cells (▲ 1.0 × 104; ● 2.5 × 104; ♦ 5.0 × 104; ▪ 1.0 × 105 per mouse) isolated from C57BL/6 donor mice. Shown are 5 animals per group from 1 of 2 independent experiments. (E) Representative photomicrographs of hematoxylin and eosin–stained sections of haired skin (×200 magnification; scale bar = 100 μ; inset ×400 magnification) and large intestines (×400 magnification; scale bar = 50 μ). The haired skin in all mice on day +10 appears essentially normal except for atrophy of subcutaneous adipose tissues (solid asterisk) in the Tcons and CD4+ iNKT groups (compared with normal adipose tissues in the bone marrow control group [open asterisk]). By day +35, the haired skin remained normal (bone marrow group) or reverted to normal (CD4+ iNKT group), but there were significant lesions in the Tcon group, with the presence of marked epidermal hyperplasia, continued atrophy of subcutaneous adipose tissues (solid asterisk), loss of hair follicles, and presence of moderate numbers of observable apoptotic keratinocytes in the stratum basale (arrows). On higher magnification (inset), these apoptotic keratinocytes appear as shrunken, hypereosinophilic (deep red) round bodies with pyknotic nuclei. The large intestines in both the bone marrow and CD4+ iNKT group appear essentially normal on days +10 and +35, except for the presence of rare apoptotic enterocytes (arrows), which appear as shrunken, hypereosinophilic (deep red) round bodies with pyknotic nuclei. In contrast, in the Tcon group, there was already development of mild lymphocytic colitis on day +10 characterized by small numbers of lymphocytes in the lamina propria (solid asterisks) that caused mild separation of intestinal glands, which themselves displayed mild loss of goblet cells with replacement by undifferentiated, proliferative basophilic (deep blue-purple) crypt enterocytes. Note the presence of small numbers of apoptotic enterocytes (arrows) as well. By day +35, the large intestinal lesions had worsened in the Tcon mice, with larger numbers of lymphocytes in the lamina propria (solid asterisks), larger separation of intestinal glands, marked loss of goblet cells with replacement undifferentiated crypt enterocytes, and larger numbers of apoptotic enterocytes (arrows). Histopathologic findings in recipient livers were mild, and only minor differences between groups were observed (not shown). †Indicates all animals from the respective group died or needed to be euthanized. Error bars indicate standard error of the mean. BM, bone marrow (control group); BMT, bone marrow transplantation.
Purified and adoptively transferred CD4+ iNKT cells protect from GVHD in a dose-dependent manner. (A) Magnetic-activated cell sorting (MACS) followed by fluorescence-activated cell sorting (FACS) of CD4+ iNKT cells. Shaded histogram curves depict CD4 isotype control. (B) Survival, (C) weight, and (D) GVHD score of BALB/c mice co-injected with 5.0 × 106 TCD-BM cells, 1.0 × 106 Tcons, and increasing doses of CD4+ iNKT cells (▲ 1.0 × 104; ● 2.5 × 104; ♦ 5.0 × 104; ▪ 1.0 × 105 per mouse) isolated from C57BL/6 donor mice. Shown are 5 animals per group from 1 of 2 independent experiments. (E) Representative photomicrographs of hematoxylin and eosin–stained sections of haired skin (×200 magnification; scale bar = 100 μ; inset ×400 magnification) and large intestines (×400 magnification; scale bar = 50 μ). The haired skin in all mice on day +10 appears essentially normal except for atrophy of subcutaneous adipose tissues (solid asterisk) in the Tcons and CD4+ iNKT groups (compared with normal adipose tissues in the bone marrow control group [open asterisk]). By day +35, the haired skin remained normal (bone marrow group) or reverted to normal (CD4+ iNKT group), but there were significant lesions in the Tcon group, with the presence of marked epidermal hyperplasia, continued atrophy of subcutaneous adipose tissues (solid asterisk), loss of hair follicles, and presence of moderate numbers of observable apoptotic keratinocytes in the stratum basale (arrows). On higher magnification (inset), these apoptotic keratinocytes appear as shrunken, hypereosinophilic (deep red) round bodies with pyknotic nuclei. The large intestines in both the bone marrow and CD4+ iNKT group appear essentially normal on days +10 and +35, except for the presence of rare apoptotic enterocytes (arrows), which appear as shrunken, hypereosinophilic (deep red) round bodies with pyknotic nuclei. In contrast, in the Tcon group, there was already development of mild lymphocytic colitis on day +10 characterized by small numbers of lymphocytes in the lamina propria (solid asterisks) that caused mild separation of intestinal glands, which themselves displayed mild loss of goblet cells with replacement by undifferentiated, proliferative basophilic (deep blue-purple) crypt enterocytes. Note the presence of small numbers of apoptotic enterocytes (arrows) as well. By day +35, the large intestinal lesions had worsened in the Tcon mice, with larger numbers of lymphocytes in the lamina propria (solid asterisks), larger separation of intestinal glands, marked loss of goblet cells with replacement undifferentiated crypt enterocytes, and larger numbers of apoptotic enterocytes (arrows). Histopathologic findings in recipient livers were mild, and only minor differences between groups were observed (not shown). †Indicates all animals from the respective group died or needed to be euthanized. Error bars indicate standard error of the mean. BM, bone marrow (control group); BMT, bone marrow transplantation.
CD4+ iNKT cells inhibit proliferation of alloreactive T cells
Previous studies have demonstrated that GVHD initiation is a precisely orchestrated process that involves priming of multiple sites in secondary lymphoid organs.14,15 In this context, we examined how adoptively transferred donor CD4+ iNKT cells affect alloreactive Tcons. First, the proliferative capacity of expanding luc+ Tcons from C57BL/6 mice was assessed. BALB/c recipient mice were injected with 1.0 × 106luc+ Tcons together with increasing numbers of CD4+ iNKT cells and 5.0 × 106 TCD-BM cells from C57BL/6 WT animals. Bioluminescence signal intensity decreased significantly during GVHD initiation in secondary lymphoid organs and GVHD target sites in animals co-injected with 2.5 × 104 and 1.0 × 105 CD4+ iNKT cells (Figure 2A-B). Next, CFSE-labeled Thy1.1+ Tcons were re-isolated from secondary lymphoid organs on day +3. Animals treated with 5.0 × 104 CD4+ iNKT cells had a significantly lower percentage of proliferating Tcons (Figure 2C).
CD4+ iNKT cells inhibit proliferation and activation of alloreactive T cells. (A) Representative bioluminescence images during GVHD initiation (day +5, upper row) and affection of GVHD target sites (day +22, lower row). Bioluminescence signal derived from luc+ Tcons injected on day 0. (B) Bioluminescence signals throughout the experiment. Shown are 5 animals per group from 1 of 2 independent experiments. (C) Graphs depict the percentage of proliferating Thy1.1+ T cells from different secondary lymphoid organs on day +3 as measured by CFSE proliferation assay. Shown is 1 of 3 independent experiments performed in triplicate. (D) Absolute number of live Thy1.1+CD44high T cells re-isolated from BALB/c recipient animals on day +6. Shown is 1 of 2 independent experiments performed in triplicate. (E) Expression of CD25 on live Thy1.1+CD44high T cells. Gates were set on isotype controls. One representative example per group of 3 mice from 1 of 3 independent experiments is shown. †Indicates all animals from the respective group died or needed to be euthanized. Error bars indicate standard error of the mean. LVR, liver; mLN, mesenteric lymph nodes; pLN, peripheral lymph nodes; SPN, spleen. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CD4+ iNKT cells inhibit proliferation and activation of alloreactive T cells. (A) Representative bioluminescence images during GVHD initiation (day +5, upper row) and affection of GVHD target sites (day +22, lower row). Bioluminescence signal derived from luc+ Tcons injected on day 0. (B) Bioluminescence signals throughout the experiment. Shown are 5 animals per group from 1 of 2 independent experiments. (C) Graphs depict the percentage of proliferating Thy1.1+ T cells from different secondary lymphoid organs on day +3 as measured by CFSE proliferation assay. Shown is 1 of 3 independent experiments performed in triplicate. (D) Absolute number of live Thy1.1+CD44high T cells re-isolated from BALB/c recipient animals on day +6. Shown is 1 of 2 independent experiments performed in triplicate. (E) Expression of CD25 on live Thy1.1+CD44high T cells. Gates were set on isotype controls. One representative example per group of 3 mice from 1 of 3 independent experiments is shown. †Indicates all animals from the respective group died or needed to be euthanized. Error bars indicate standard error of the mean. LVR, liver; mLN, mesenteric lymph nodes; pLN, peripheral lymph nodes; SPN, spleen. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CD4+ iNKT cells prevent activation of alloreactive T cells
We investigated whether CD4+ iNKT cells inhibit activation of donor T cells in vivo. On day +6, a similar percentage of CD44high effector cells among all Thy1.1+ Tcons from secondary lymphoid organs was observed in animals that received Tcons only compared with Tcons with CD4+ iNKT cells. In contrast, the absolute number of Thy1.1+CD44high effector cells was significantly lower, consistent with the finding of decreased proliferation (Figure 2D). On day +6, the expression of IL-2R α-chain (CD25) of Thy1.1+ Tcons as well as their CD4 and CD8 subsets was significantly decreased, which reflects suppression of activation of alloreactive T cells in the presence of adoptively transferred donor CD4+ iNKT cells (Figure 2E).
CD4+ iNKT cells promote a Th2-biased cytokine pattern
GVHD is characterized by an expansion of alloreactive T cells that secrete large amounts of proinflammatory Th1-biased cytokines such as interferon gamma (IFN-γ), thus perpetuating the inflammatory cycle of alloreactivity. On day +10 following HCT, donor T cells were analyzed for production of several different cytokines known to play a role in GVHD pathophysiology. Live Thy1.1+FoxP3–CD4+ T cells from CD4+ iNKT cell-treated mice showed a Th2-biased cytokine pattern characterized by a lower expression of mIFN-γ and higher expression of mIL-5 compared with Tcon-only control animals (Figure 3A). In addition, sera from animals that were treated with 5.0 × 104 CD4+ iNKT cells had significantly higher concentrations of the Th2 cytokines murine IL-4 (mIL-4), mIL-5, and mIL-6, whereas serum concentrations of murine tumor necrosis factor α and mIFN-γ were decreased compared with animals that received Tcons only (Figure 3B). We did not find significant differences for mIL-10.
CD4+ iNKT cells promote a Th2-biased cytokine response in vivo. (A) mIFN-γ and mIL-5 staining of live Thy1.1+FoxP3–CD4+ T cells. Gates were set on isotype controls. Shown are representative dot plots from 1 of 2 independent experiments. (B) Serum levels of cytokines in the presence of Tcon or Tcon + CD4+ iNKT cells. Shown are 3 animals per group from 1 of 3 independent experiments. Error bars indicate standard error of the mean. mTNF-α, murine tumor necrosis factor α. *P ≤ .05; **P ≤ .01.
CD4+ iNKT cells promote a Th2-biased cytokine response in vivo. (A) mIFN-γ and mIL-5 staining of live Thy1.1+FoxP3–CD4+ T cells. Gates were set on isotype controls. Shown are representative dot plots from 1 of 2 independent experiments. (B) Serum levels of cytokines in the presence of Tcon or Tcon + CD4+ iNKT cells. Shown are 3 animals per group from 1 of 3 independent experiments. Error bars indicate standard error of the mean. mTNF-α, murine tumor necrosis factor α. *P ≤ .05; **P ≤ .01.
CD4+ iNKT cells promote an expansion of donor Tregs
Because it has been reported that host iNKT cells induce an expansion of donor Tregs in the setting of TLI/ATG conditioning,16 we hypothesized that donor CD4+ iNKT cells promote the expansion of donor Tregs after myeloablative conditioning with TBI. BALB/c recipient mice were injected with 1.0 × 106 Thy1.1+ Tcons together with TCD-BM with or without 5.0 × 104 CD4+ iNKT cells. A significantly higher percentage and absolute cell number of Thy1.1+ Tregs among donor alloreactive CD4+ T cells was observed in secondary lymphoid organs and GVHD target sites of BALB/c recipient mice on day +10 (Figure 4A-C). In addition, we investigated the impact of donor CD4+ iNKT cells on host Tregs. We found splenic host Tregs at a higher percentage (12.1% vs 0.7%; P = .004) and absolute cell number (2720 vs 22; P = .048) in animals treated with CD4+ iNKT cells. However, no significant difference was found for host Tregs from lymph nodes and GVHD target tissues compared with the Tcon control group. Because of higher cell numbers, we concluded that donor Tregs play a predominant role in protection from GVHD compared with host Tregs.
CD4+ iNKT cells promote an expansion of donor Tregs. (A) FoxP3 expression of live Thy1.1+CD4+ T cells. Gates are set on isotype controls. Shown are representative dot plots from 1 of at least 5 independent experiments. (B) Relative (percentage of live Thy1.1+CD4+) and (C) absolute cell numbers of FoxP3-expressing donor CD4+ T cells. Shown are 3 animals per group from 1 of at least 5 independent experiments. (D) Expression of CD25, cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3 (Lag3), and transforming growth factor β (latency-associated peptide) (TGF-β [LAP]) on live Thy1.1+CD4+FoxP3+ T cells. Shaded curves depict isotype controls. Shown are pooled data of 3 mice from 1 of at least 2 independent experiments. (E) Mixed lymphocyte reaction (MLR) of donor Thy1.1+ Tregs pooled and sorted from BALB/c mice, donor Tcon effectors, and irradiated allogeneic BALB/c stimulators. The MLR was performed in triplicate. Shown is 1 of 3 independent experiments. Error bars indicate standard error of the mean. E, effector (Tcon); I, inhibitor (Thy1.1+ Treg); ITN, intestine. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
CD4+ iNKT cells promote an expansion of donor Tregs. (A) FoxP3 expression of live Thy1.1+CD4+ T cells. Gates are set on isotype controls. Shown are representative dot plots from 1 of at least 5 independent experiments. (B) Relative (percentage of live Thy1.1+CD4+) and (C) absolute cell numbers of FoxP3-expressing donor CD4+ T cells. Shown are 3 animals per group from 1 of at least 5 independent experiments. (D) Expression of CD25, cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3 (Lag3), and transforming growth factor β (latency-associated peptide) (TGF-β [LAP]) on live Thy1.1+CD4+FoxP3+ T cells. Shaded curves depict isotype controls. Shown are pooled data of 3 mice from 1 of at least 2 independent experiments. (E) Mixed lymphocyte reaction (MLR) of donor Thy1.1+ Tregs pooled and sorted from BALB/c mice, donor Tcon effectors, and irradiated allogeneic BALB/c stimulators. The MLR was performed in triplicate. Shown is 1 of 3 independent experiments. Error bars indicate standard error of the mean. E, effector (Tcon); I, inhibitor (Thy1.1+ Treg); ITN, intestine. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Re-isolated Tregs display an activated phenotype and suppress the mixed lymphocyte reaction
Next, the phenotype of Thy1.1+ Tregs from CD4+ iNKT cell–treated recipient mice was analyzed. Upregulation of CD25, CTLA-4, PD-1, Lag3, and TGF-β (LAP) was observed that was comparable to Tregs re-isolated from Tcon-only control animals (Figure 4D). To determine Treg suppressive function in vitro, pooled purified donor Thy1.1+ Tregs from secondary lymphoid organs and livers of animals treated with CD4+ iNKT cells were assessed functionally on day +10 after HCT for their ability to inhibit T-cell proliferation by mixed lymphocyte reaction. We found that increasing doses of donor Tregs efficiently suppress proliferation of Tcons when challenged with allogeneic irradiated splenocytes from BALB/c mice (P = .04) (Figure 4E).
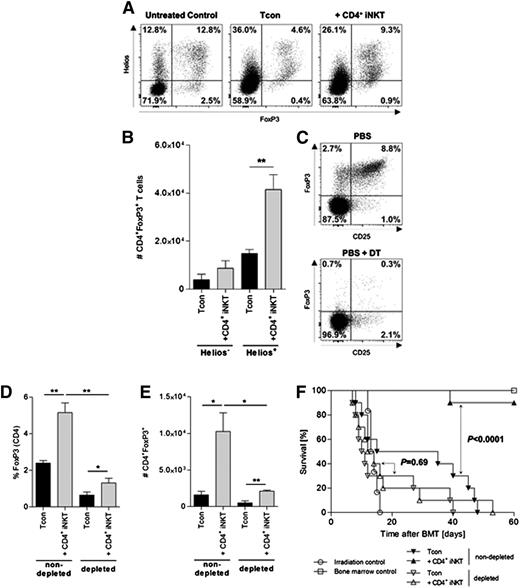
Tregs expand from the Tcon inoculum and are required for protection from GVHD
We tested whether Tregs from CD4+ iNKT cell–treated mice expand from the Tcon inoculum or whether they were induced Tregs (iTregs). The intranuclear transcription factor Helios is mainly expressed in naturally occurring thymus-derived Tregs (nTregs) and has been proposed to distinguish between nTregs and iTregs.17,18 The majority of Tregs re-isolated from secondary lymphoid organs and GVHD target sites of CD4+ iNKT cell–treated mice express Helios (Figure 5A). In addition, a significant increase in the total number of donor Tregs in CD4+ iNKT cell–treated animals was observed only for Helios-expressing cells (Figure 5B). Thus, we hypothesized that depletion of Tregs from the graft would result in a decreased expansion of donor Tregs. To test this hypothesis, FoxP3DTR C57BL/6 mice were injected intraperitoneally with diphtheria toxin prior to harvest, which resulted in a marked reduction of FoxP3-expressing cells among CD4+ T cells (<1%; Figure 5C). BALB/c recipient mice received grafts that were either depleted or nondepleted of Tregs with or without adoptive transfer of CD4+ iNKT cells. Animals that received Treg-depleted grafts failed to show an expansion of Tregs in contrast to animals that received nondepleted grafts when treated with CD4+ iNKT cells (P = .002) (Figure 5D-E). Interestingly, a significant increase of donor Tregs through CD4+ iNKT cells could be observed on a lower level in animals receiving Treg-depleted grafts, which supports the influence of CD4+ iNKT cells on Tregs already contained in the graft (Figure 5D-E). In addition, depletion of Tregs from the graft resulted in a loss of survival benefit mediated through adoptive transfer of donor CD4+ iNKT cells. BALB/c mice that received a graft depleted of Tregs and were treated with CD4+ iNKT cells showed no significant improvement in survival compared with the Tcon-only control group (P = .69). In contrast, adoptively transferred CD4+ iNKT cells protected from GVHD lethality when mice received a Treg-nondepleted graft (P < .0001) (Figure 5F). To test the hypothesis that Treg-depleted grafts may confer more aggressive GVHD that cannot be overcome by CD4+ iNKT cells, we tested alternative Tcon and Treg dosing strategies. By using a reduced number of Tcons (7.5 × 105) to compensate for the potential of enhanced GVHD and increasing the number of CD4+ iNKT cells (1.0 × 105), we did not observe protection from GVHD in animals that received grafts depleted of donor Tregs compared with animals that received a Treg-nondepleted graft in which CD4+ iNKT cells did protect from GVHD lethality (data not shown). Our results demonstrate that CD4+ iNKT cells lead to an expansion of donor Tregs from the Tcon inoculum and that these Tregs are required to protect from GVHD lethality.
Tregs expand from the Tcon inoculum and are required for protection from GVHD. (A) Expression of FoxP3 and Helios in live splenic Thy1.1+CD4+ T cells. Shown are representative dot plots from 1 of 3 independent experiments. (B) Total numbers of live splenic Helios–FoxP3+ and Helios+FoxP3+ Thy1.1+CD4+ T cells. Shown is 1 of 3 independent experiments. (C) Expression of CD25 and FoxP3 in live CD4+ T cells after intraperitoneal injection of 50 µg⋅kg−1 diphtheria toxin (DT) into FoxP3DTR C57BL/6 donor mice on 2 consecutive days. Shown are representative dot plots from 1 of 2 independent experiments. (D) Relative and (E) absolute numbers of donor Thy1.1+ Tregs re-isolated from BALB/c recipient mice that received either Treg-nondepleted (left bars) or Treg-depleted grafts (right bars), respectively. Population gated on live Thy1.1+CD4+ T cells. Shown are 3 animals per group from 1 of 2 independent experiments. (F) Pooled survival data from 2 independent experiments of BALB/c mice treated with CD4+ iNKT cells in the presence and absence of donor Tregs. Ten animals per group except irradiation control (n = 6). Error bars indicate standard error of the mean. *P ≤ .05; **P ≤ .01.
Tregs expand from the Tcon inoculum and are required for protection from GVHD. (A) Expression of FoxP3 and Helios in live splenic Thy1.1+CD4+ T cells. Shown are representative dot plots from 1 of 3 independent experiments. (B) Total numbers of live splenic Helios–FoxP3+ and Helios+FoxP3+ Thy1.1+CD4+ T cells. Shown is 1 of 3 independent experiments. (C) Expression of CD25 and FoxP3 in live CD4+ T cells after intraperitoneal injection of 50 µg⋅kg−1 diphtheria toxin (DT) into FoxP3DTR C57BL/6 donor mice on 2 consecutive days. Shown are representative dot plots from 1 of 2 independent experiments. (D) Relative and (E) absolute numbers of donor Thy1.1+ Tregs re-isolated from BALB/c recipient mice that received either Treg-nondepleted (left bars) or Treg-depleted grafts (right bars), respectively. Population gated on live Thy1.1+CD4+ T cells. Shown are 3 animals per group from 1 of 2 independent experiments. (F) Pooled survival data from 2 independent experiments of BALB/c mice treated with CD4+ iNKT cells in the presence and absence of donor Tregs. Ten animals per group except irradiation control (n = 6). Error bars indicate standard error of the mean. *P ≤ .05; **P ≤ .01.
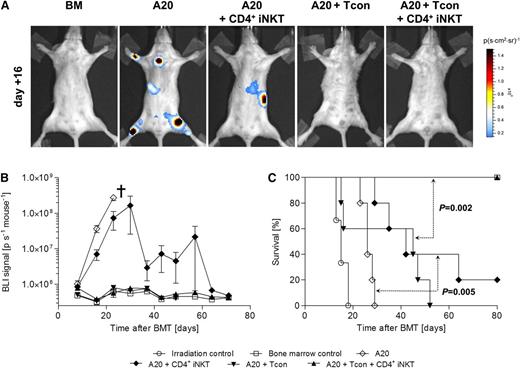
CD4+ iNKT preserve Tcon-mediated GVT reactions
GVT activity is mediated through alloreactive T cells. Thus, we tested whether CD4+ iNKT cells abrogate GVT reactions. The tail vein was injected with 1.0 × 104luc+ A20 cells after TBI on day 0. All animals receiving TCD-BM together with luc+ A20 cells showed steadily increasing bioluminescence signals and eventually died of progressive lymphoma. Mice that received luc+ A20 cells together with 1.0 × 106 Tcons all died of GVHD without evidence of lymphoma, whereas animals treated with Tcons together with 5.0 × 104 CD4+ iNKT cells survived throughout the whole experiment without evidence of lymphoma (P = .002). Interestingly, BALB/c mice that received 5.0 × 104 CD4+ iNKT cells without Tcons showed significantly lower bioluminescence signals on days +16 and +23 (P = .008 and P = .01, respectively) and a significant survival benefit compared with their A20 control group (P = .005) (Figure 6A-C). We conclude from these findings that CD4+ iNKT cells preserved Tcon-mediated GVT reactions and exerted GVT effects by themselves following allogeneic HCT. Furthermore, a second tumor model using BCL1 lymphoma cells was studied in a similar design. BALB/c recipient mice were injected with 3000 luc+ BCL1 cells 7 days prior to HCT. Tumor engraftment was verified by BLI on day −1. We observed a steady increase of signal intensity derived from luc+ BCL1 cells in animals treated with TCD-BM only or TCD-BM together with CD4+ iNKT cells; all animals died of lymphoma, and no difference in survival was observed between groups (P = .44). Animals that were treated with 1.0 × 106 Tcons all died of GVHD without evidence of lymphoma as measured by BLI. In contrast, all animals that received Tcons together with CD4+ iNKT cells survived without evidence of lymphoma (P =.002) (supplemental Figure 2A-C).
CD4+ iNKT cells preserve Tcon-mediated GVT reactions against A20 lymphoma cells. (A) Tumor growth of luc+ A20 cells was assessed by BLI. Shown are representative bioluminescence images of day +16. (B) Bioluminescence signal intensity and (C) survival of BALB/c mice throughout the whole experiment. Shown is 1 of 2 independent experiments with 5 animals per group except irradiation control (n = 3). †Indicates all animals from the respective group died or needed to be euthanized. Error bars indicate standard error of the mean.
CD4+ iNKT cells preserve Tcon-mediated GVT reactions against A20 lymphoma cells. (A) Tumor growth of luc+ A20 cells was assessed by BLI. Shown are representative bioluminescence images of day +16. (B) Bioluminescence signal intensity and (C) survival of BALB/c mice throughout the whole experiment. Shown is 1 of 2 independent experiments with 5 animals per group except irradiation control (n = 3). †Indicates all animals from the respective group died or needed to be euthanized. Error bars indicate standard error of the mean.
Discussion
iNKT cells are a rare cell population characterized through the expression of the semi-invariant TCRα-chain Vα24-Jα18 in humans and Vα14-Jα18 in mice.6,7 Their functional hallmark is the rapid release of immunoregulatory cytokines upon stimulation, thus giving these cells a phenotype of innate-like lymphocytes.6,7 Host iNKT cells are crucial mediators of tolerance after reduced-intensity conditioning with TLI/ATG.8,16,19 In this study, we show that low numbers of highly purified and adoptively transferred donor CD4+ iNKT cells protect from GVHD morbidity and lethality in a dose-dependent manner while preserving Tcon-mediated GVT effects. CD4+ iNKT cells inhibit Tcon proliferation and upregulation of CD25 during the critical phase of GVHD initiation in secondary lymphoid organs, meanwhile promoting a robust expansion of fully functional donor Tregs. We found that these Tregs express the nuclear transcription factor Helios and expand from Tregs contained within the graft. Depletion of Tregs from the graft abrogated the expansion of donor Tregs and resulted in a loss of protection from GVHD lethality.
Our group recently described a different subset of NKT cells that were also capable of suppressing GVHD. We showed that adoptively transferred donor CD4+CD49b+ NKT cells provide protection from GVHD in an IL-4–dependent manner.20 In contrast to this study, low doses of CD4+CD49b+ NKT cells did not inhibit proliferation of Tcons but did decrease the production of proinflammatory cytokines secreted by alloreactive T cells. Low doses of CD4+CD49b+ NKT cells were effective in preventing GVHD, which is similar to our results. These findings indicate that different NKT cell subsets use distinct cellular and/or humoral mechanisms to overcome Tcon-mediated alloreactivity and reflect the complex functional diversity of NKT cells.21
Pillai et al16 showed that host iNKT cells promote an IL-4–dependent expansion of donor Tregs and that host iNKT and donor Tregs are both required for protection from GVHD in the setting of TLI/ATG conditioning. GVHD protection was lost in NKT-cell–deficient hosts (Jα18−/−) or when the donor transplant was depleted of Tregs. Host iNKT cells can mediate their immunologic effects because of their relative radioresistance fulfilled with TLI/ATG conditioning. This concept of tolerance induction has been successfully applied in combined allogeneic HCT and kidney transplantation.22,23 In this study, we showed for the first time that adoptively transferred donor CD4+ iNKT cells are promoting a robust expansion of donor Tregs required for protection from GVHD. These findings have significant clinical importance because graft manipulation is not restricted to a particular type of conditioning regimen, and different protocols for culturing and/or activating murine and human iNKT cells are available to expand this rare but potent immunoregulatory cell population.24-27 In this context, our study shows that CD4+ iNKT:Tcon ratios as low as 1:100 are sufficient to provide robust protection from GVHD in murine models. In contrast, our previous studies showed that rather high numbers of adoptively transferred donor Tregs were required (Treg:Tcon ratio 1:1 or 1:2), although this ratio could be decreased with a delayed administration of Tcons after allogeneic HCT resulting in an advantageous in vivo expansion of Tregs.15 We found expression of the Ikaros transcription factor Helios in Tregs from CD4+ iNKT cell–treated BALB/c recipient mice indicating that donor CD4+ iNKT cells promote expansion of Tregs contained within the graft rather than inducing Tregs from the naïve T-cell compartment. Depletion of Tregs from the graft abrogated Treg expansion and the survival benefit observed by the adoptive transfer of CD4+ iNKT cells.
Because allogeneic HCT is the most widely applied and most advanced type of cellular immunotherapy for the treatment of otherwise fatal hematologic malignancies, we investigated the influence of CD4+ iNKT cells on Tcon-mediated GVT effects. In this study, augmenting Tcon grafts with CD4+ iNKT cells preserved GVT effects in 2 tumor models. Although we did not find a significant difference in luc+ BCL1 tumor clearance in BALB/c recipient mice treated with CD4+ iNKT cells only compared with control animals, we did observe significantly decreased bioluminescence signal intensities and a significant survival benefit in BALB/c recipient mice that received luc+ A20 lymphoma cells, suggesting that, in some circumstances, CD4+ iNKT cells have modest antitumor activity. Therefore, CD4+ iNKT cells do not abrogate Tcon-mediated GVT effects but may be able to exert distinct antitumor activity by themselves in the setting of allogeneic HCT. iNKT cells mediate tumor control through the granzyme B/perforin and Fas/FasL pathways.28,29 In humans, high iNKT cell numbers or activation of iNKT cells through glycolipids correlates with an improved outcome in different types of cancer.30-34
Recent retrospective clinical data strongly support the beneficial immunologic effects of donor iNKT cells in the setting of allogeneic HCT in humans. Chaidos et al35 showed in a multivariate analysis that a higher graft iNKT cell dose was associated with a significantly lower risk of acute GVHD. Similarly, Rubio et al36 found that low peripheral blood iNKT:T-cell ratios posttransplant were an independent factor associated with the occurrence of acute GVHD. Taken together, iNKT cells play a central role in promoting tolerance following allogeneic bone marrow transplantation in humans. Cellular immunotherapy with iNKT cells might harbor some benefits compared with the adoptive transfer of Tregs. In previous clinical trials, Tregs were characterized by the expression of surface molecules because intracellular staining for nuclear transcription factor FoxP3 is not feasible in the context of adoptive transfer of live cells.3,4 In contrast, iNKT cells are clearly defined through their semi-invariant TCR, allowing the isolation of a pure cell population. Depending on the protocol, the in vitro expansion capacity of iNKT cells is several thousand fold and thus is much higher than that of Tregs.24-27 iNKT cells can be expanded easily from donor peripheral blood, whereas apheresis eventually followed by in vitro expansion is required for Tregs. Moreover, we showed that much lower iNKT cells doses were necessary to confer protection from GVHD lethality compared with Tregs. iNKT cells may also contribute to GVT effects as shown in Figure 6 and supplementary Figure 2, whereas no such antitumor effects have been observed for Tregs alone.2 However, further studies are required to investigate the impact of posttransplant immunosuppressive drugs on donor iNKT cells.
In conclusion, low numbers of highly purified and adoptively transferred CD4+ iNKT cells protect from GVHD morbidity and mortality through an expansion of donor Tregs while retaining GVT effects. Despite the fact that human iNKT cells are a rare cell population, the robust in vivo activity at low cell numbers and feasibility of in vitro expansion and activation provides the basis for clinical translation. Adoptive transfer of iNKT cells is an appealing approach to overcome alloimmunity after allogeneic HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kent P. Jensen from the Strober Laboratory for assistance with the Luminex multiplex assay. PBS-57-loaded mCD1d tetramer was provided by the National Institutes of Health Tetramer Facility.
This work was supported in part by National Institutes of Health Program Project Grants, National Cancer Institute CA49605 and National Heart, Lung, and Blood Institute HL075462, the Dr. Mildred Scheel Stiftung (D.S., C.B.), and the Fondazione Italiana per la Ricerca sul Cancro (A.P.).
Authorship
Contributions: D.S. designed and performed research, analyzed data and wrote the manuscript; A.P., M.A., Y.P., J.B., and C.B. performed research; R.H.L. evaluated histopathology and took photomicrographs; and E.H.M. and R.S.N. are senior co-authors of this study and provided overall guidance. All authors edited the manuscript for content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Sciences Research Building, Room 2205, 269 Campus Dr, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
Author notes
E.H.M. and R.S.N. contributed equally to this manuscript.

![Figure 1. Purified and adoptively transferred CD4+ iNKT cells protect from GVHD in a dose-dependent manner. (A) Magnetic-activated cell sorting (MACS) followed by fluorescence-activated cell sorting (FACS) of CD4+ iNKT cells. Shaded histogram curves depict CD4 isotype control. (B) Survival, (C) weight, and (D) GVHD score of BALB/c mice co-injected with 5.0 × 106 TCD-BM cells, 1.0 × 106 Tcons, and increasing doses of CD4+ iNKT cells (▲ 1.0 × 104; ● 2.5 × 104; ♦ 5.0 × 104; ▪ 1.0 × 105 per mouse) isolated from C57BL/6 donor mice. Shown are 5 animals per group from 1 of 2 independent experiments. (E) Representative photomicrographs of hematoxylin and eosin–stained sections of haired skin (×200 magnification; scale bar = 100 μ; inset ×400 magnification) and large intestines (×400 magnification; scale bar = 50 μ). The haired skin in all mice on day +10 appears essentially normal except for atrophy of subcutaneous adipose tissues (solid asterisk) in the Tcons and CD4+ iNKT groups (compared with normal adipose tissues in the bone marrow control group [open asterisk]). By day +35, the haired skin remained normal (bone marrow group) or reverted to normal (CD4+ iNKT group), but there were significant lesions in the Tcon group, with the presence of marked epidermal hyperplasia, continued atrophy of subcutaneous adipose tissues (solid asterisk), loss of hair follicles, and presence of moderate numbers of observable apoptotic keratinocytes in the stratum basale (arrows). On higher magnification (inset), these apoptotic keratinocytes appear as shrunken, hypereosinophilic (deep red) round bodies with pyknotic nuclei. The large intestines in both the bone marrow and CD4+ iNKT group appear essentially normal on days +10 and +35, except for the presence of rare apoptotic enterocytes (arrows), which appear as shrunken, hypereosinophilic (deep red) round bodies with pyknotic nuclei. In contrast, in the Tcon group, there was already development of mild lymphocytic colitis on day +10 characterized by small numbers of lymphocytes in the lamina propria (solid asterisks) that caused mild separation of intestinal glands, which themselves displayed mild loss of goblet cells with replacement by undifferentiated, proliferative basophilic (deep blue-purple) crypt enterocytes. Note the presence of small numbers of apoptotic enterocytes (arrows) as well. By day +35, the large intestinal lesions had worsened in the Tcon mice, with larger numbers of lymphocytes in the lamina propria (solid asterisks), larger separation of intestinal glands, marked loss of goblet cells with replacement undifferentiated crypt enterocytes, and larger numbers of apoptotic enterocytes (arrows). Histopathologic findings in recipient livers were mild, and only minor differences between groups were observed (not shown). †Indicates all animals from the respective group died or needed to be euthanized. Error bars indicate standard error of the mean. BM, bone marrow (control group); BMT, bone marrow transplantation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/22/10.1182_blood-2014-05-576017/4/m_3320f1.jpeg?Expires=1770990586&Signature=PxEaGRlcEFDexjny3XpZtj6OdTrycs70OpzkoRgcufRS2MvT2-WtM75IopKzGJsHZ3nlvu83kPGGL9p4hLMGyEzOUHkuUvB3gKFeZ9QETaPJlBEVJEqOf03z9GPVKo9~10XVPAxMAy2wM2vZDf3ZvHxYACUv9oYNVhpMeadb97zqY~Cwij-uV5Pu6Yfrj0gvdqo3DguP5OcVW5GKWetAwGUF1XKpmCt-exfUwCi7XF1YGjsf~fTOtcgbwv8a5BHqGc29I3tHBuK9cCBAO03vZpJ5NDkuZ0SdIYRV8O91pl~cgt4Tc5wzAiATeoq2QPa2ofbVLve6qJyz6LqPHDXYeg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 4. CD4+ iNKT cells promote an expansion of donor Tregs. (A) FoxP3 expression of live Thy1.1+CD4+ T cells. Gates are set on isotype controls. Shown are representative dot plots from 1 of at least 5 independent experiments. (B) Relative (percentage of live Thy1.1+CD4+) and (C) absolute cell numbers of FoxP3-expressing donor CD4+ T cells. Shown are 3 animals per group from 1 of at least 5 independent experiments. (D) Expression of CD25, cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3 (Lag3), and transforming growth factor β (latency-associated peptide) (TGF-β [LAP]) on live Thy1.1+CD4+FoxP3+ T cells. Shaded curves depict isotype controls. Shown are pooled data of 3 mice from 1 of at least 2 independent experiments. (E) Mixed lymphocyte reaction (MLR) of donor Thy1.1+ Tregs pooled and sorted from BALB/c mice, donor Tcon effectors, and irradiated allogeneic BALB/c stimulators. The MLR was performed in triplicate. Shown is 1 of 3 independent experiments. Error bars indicate standard error of the mean. E, effector (Tcon); I, inhibitor (Thy1.1+ Treg); ITN, intestine. *P ≤ .05; **P ≤ .01; ***P ≤ .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/22/10.1182_blood-2014-05-576017/4/m_3320f4.jpeg?Expires=1770990586&Signature=dMEp3Ybi-Cc-A2x3AmqHJ8~vQ1ixAjsco4ta1jo6ThzR9KgewibRmwWwAVYyMWKBonSQrLO7FxVAPtnt3~BI07~aJhNH7FG34BkxV1O~eUyEQlbaC6gSwWhOsFnaLcCrG-l1owDwFmNJI~wljl-s65vhfXVir9rwSTYUQga2e6D5g4g4fBvsMjyy2hP87sFArxp607GgtySnT7vab4ufSpumHmwafsAc6i0S~9U9LOY3q4PHP4vzPY7lujm7vCXScVZvNgbttHnvHsg0Z57R3RRse2RAHFN0bupN8HpNoMYE2393xGCMv-77ohVmNatw2O70KDpMyWWmAZgeIW4zLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)