Key Points
Sustained responses to steroids in patients with GVHD are close to 50%.
The addition of mycophenolate mofetil to steroids did not improve clinical outcome over steroids alone.
Abstract
Corticosteroids are the accepted primary therapy for acute graft-versus-host disease (GVHD), but durable responses are seen in only about half of the patients. Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0802, a phase 3 multicenter randomized double-blinded trial, was designed to test whether mycophenolate mofetil (MMF) plus corticosteroids was superior to corticosteroids alone as initial therapy for acute GVHD. Patients with newly diagnosed acute GVHD were eligible if they required systemic therapy. Patients were randomized to receive prednisone with either MMF or placebo. The primary end point was acute or chronic GVHD-free survival at day 56 after initiation of therapy. A futility rule for GVHD-free survival at day 56 was met at a planned interim analysis after 235 patients (of 372) were enrolled: 116 MMF, 119 placebo. Baseline characteristics were well balanced between treatment groups including grade and organ distribution of GVHD. GVHD-free survival at day 56, cumulative incidence of chronic GVHD at 12 months, overall survival, Epstein-Barr virus reactivation, severe, life-threatening infections, relapse at 12 months, and quality of life were similar. The addition of MMF to corticosteroids as initial therapy for acute GVHD does not improve GVHD-free survival compared with corticosteroids alone. This trial was registered at www.clinicaltrials.gov as #NCT01002742.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3335.
Disclosures
Robert S. Negrin, Associate Editor, served as an advisor or consultant for Bluebird Biologics and Jazz Pharmaceuticals, Inc. Also, he owns stock, stock options, or bonds from Concentrx Pharmaceuticals. The authors and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Compare the effects of mycophenolate mofetil plus corticosteroids vs corticosteroids alone as initial treatment of acute graft-versus-host disease (GVHD) on the primary endpoint of acute or chronic GVHD-free survival at day 56 after therapy initiation, based on a phase 3, multicenter, randomized, double-blinded trial.
Compare the effects of mycophenolate mofetil plus corticosteroids vs corticosteroids alone as initial treatment of acute GVHD on secondary endpoints.
Compare the toxicities of mycophenolate mofetil plus corticosteroids vs corticosteroids alone as initial treatment of acute GVHD.
Release date: November 20, 2014; Expiration date: November 20, 2015
Introduction
Acute graft-versus-host disease (aGVHD) is a common complication of allogeneic hematopoietic stem cell transplantation (HSCT), with an expected incidence of up to 50% or higher.1-3 Standard first-line therapy for the treatment of aGVHD involves corticosteroids, usually prednisone at a dose of 2 mg/kg per day.3 Despite initial responses, fewer than half of patients have durable complete responses.2,4,5 Because patients who do not respond or progress after an initial response have high mortality, there is great interest in adding agents to corticosteroids as initial therapy to improve outcomes. To date, prospective randomized clinical trials combining antithymocyte globulin (ATG) or monoclonal antibodies with corticosteroids as initial therapy have not demonstrated benefit compared with corticosteroids alone.4,6-9
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducted a randomized phase 2 study (BMT CTN 0302) to assess the response rates of 4 drugs (pentostatin, mycophenolate mofetil [MMF], denileukin diftitox, and etanercept) in combination with corticosteroids as initial therapy for aGVHD.3 MMF was found to be the most promising of the 4 agents tested, with the combination of MMF/corticosteroids achieving 60% complete response by day 28, 9-month overall survival (OS) of 64%, and GVHD-free survival by day 56 of 71%.3 Upon this platform, the BMT CTN launched study 0802, a phase 3 randomized, multicenter, double-blinded placebo-controlled study comparing corticosteroids/MMF with corticosteroids/placebo as first-line therapy for aGVHD.
Methods
Patients
Adult and pediatric allogeneic HSCT recipients with any grade newly diagnosed aGVHD deemed clinically to require systemic corticosteroid therapy were eligible. There was no restriction on graft source. Absolute neutrophil count (ANC) ≥500 cells per μL without growth factors was required for study entry. Patients who developed aGVHD after disease relapse or unplanned donor lymphocyte infusion were excluded. Biopsy of the organs involved was not required for the diagnosis of aGVHD. Patients must have received no previous systemic immune suppressive therapy for the treatment of aGVHD except for a maximum 72 hours of prior corticosteroid therapy at >0.5 mg/kg methylprednisolone or equivalent. Patients must be able to swallow MMF/placebo capsules, and have a clinical status at enrolment to allow tapering of corticosteroids to not less than 0.25 mg/kg per day prednisone (0.2 mg/kg per day methylprednisolone) at day 28 of therapy. Patients receiving MMF or mycophenolic acid within 7 days of screening, those with chronic GVHD, hepatic veno-occlusive disease, or renal failure on dialysis were not eligible. Patients were enrolled and treated within 72 hours of making the determination that the GVHD required therapy.
Randomization
After registration and enrollment, study participants were randomized in a 1:1 fashion to receive either MMF or placebo using random block sizes. Randomization was stratified by transplant center and grade of aGVHD (I-II vs III-IV) at study entry.
Treatment plan
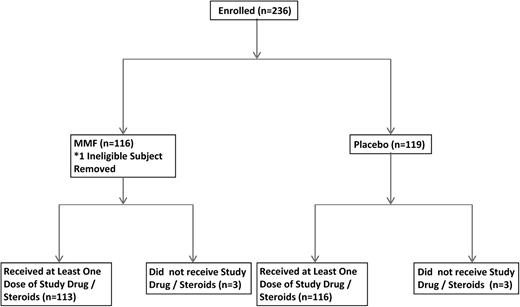
All patients received corticosteroids at a dose of prednisone 2 mg/kg per day orally (or methylprednisolone 1.6 mg/kg per day IV) along with assigned MMF/placebo (Figure 1). Corticosteroid taper followed local institutional practice but the taper could not commence sooner than 3 days after randomization and the dose could not be tapered to <0.25 mg/kg per day prednisone (or 0.2 mg/kg per day methylprednisolone) on day 28. Topical corticosteroids, including skin creams or oral nonabsorbable corticosteroids, were allowed. MMF/placebo was given as 1000 mg or 20 mg/kg (for patients <60 kg) orally or IV every 8 hours. Study drug was discontinued by day 56 or when prednisone taper was complete, whichever occurred first. Vomited doses were not replaced. The dose of MMF used in this trial was different than in BMT CTN 0302 because the pharmacokinetic analysis of patients receiving MMF on BMT CTN 0302 showed that higher exposure to MMF was associated with a better outcome.10 MMF/placebo was obtained and distributed by the BMT CTN. The drug was supplied as 250-mg capsules in bottles of 100 mg each and both were identical. The IV formulation was supplied as a lyophilized powder to be reconstituted in 5% dextrose in water. Patients in the placebo group requiring IV formulation received dextrose bags of matched volume to active and relabeled to mask the treatment.
Supportive care and antimicrobial prophylaxis followed institutional standards. Reduction to every 12-hour dosing was allowed for gastrointestinal (GI) toxicity. If symptoms persisted or were severe, the study drug could be held for 48 to 72 hours or switched to the IV formulation. Interchange from oral to IV formulation of MMF/placebo was allowed based on patient tolerance. Reduction to q12-hour dosing was also recommended if the ANC declined to <1000/μL. If the ANC dropped below 500/μL, the MMF/placebo was discontinued until neutrophil recovery occurred, and restarted at every 12-hour dosing. Use of growth factors was allowed. Administration of the study drug was discontinued permanently if administration was suspended for >14 days. If aGVHD progressed (see GVHD scoring) after at least 5 days, or if there was no response after 14 days of treatment, the study drug was discontinued, and secondary therapy may be given at the discretion of the treating physician. In the event of aGVHD flare, the dose of corticosteroids could be increased at the discretion of the treating physician as long as this increase was to <2.5 mg/kg per day of prednisone (or equivalent).
GVHD scoring and response determination
Weekly aGVHD organ stage scores, overall clinical grade, biopsy information for GVHD, and relevant differential diagnosis were recorded. aGVHD was graded by the Keystone Consensus Criteria.11 Complete response (CR) was defined as complete resolution of all signs and symptoms of aGVHD in all evaluable organs without additional therapies. A partial response (PR) was defined as improvement of 1 stage in 1 or more organs involved by GVHD without progression in others. Progression was defined as worsening in 1 or more organs by 1 or more stage without improvement in any involved organ. No response was defined as stable disease or absence of improvement in any organ involved by GVHD within 14 days of therapy initiation. A flare in GVHD was defined as any increase in symptoms or therapy for aGVHD after an initial response (CR or PR). All toxicities were reported regardless of whether they were thought to be related to the study treatment. Adverse events (AEs) were evaluated according to NCI Common Terminology Criteria for AE, version 3.0. Presence or absence of chronic GVHD (cGVHD) was recorded at days 56, 180, and 360 after randomization. The primary end point of the study was GVHD-free survival at day 56 after randomization, calculated as the proportion of patients in each arm who were alive at day 56, achieved a CR from their assigned therapy, did not experience a GVHD flare, did not need additional GVHD therapy, and did not develop cGVHD. A study end point committee, blinded to study drug assignment, reviewed and verified all response and end point data before any statistical analysis.
Quality of life
Adult study participants who were able to communicate in English completed the MD Anderson Symptom Inventory (MDASI) at enrolment prior to randomization and at day 56 ± 7 days. The MDASI is a 19-item instrument that captures 13 symptoms and 6 items measuring interference with life.12 The MDASI was scored according to the recommendations of the developers.
Statistical considerations
The primary analysis was a comparison of the GVHD-free survival probabilities at day 56 after randomization based on intention to treat. The study was designed to enroll 372 patients (186 in each arm) to have 80% power to detect a 15% difference in the GVHD-free survival probabilities (from 47% to 62%), after accounting for efficacy and futility interim analyses using an O’Brien-Fleming–type boundary for both type I and type II errors. This plan was based on a 2-sample Z test for comparing proportions with a 2-sided α of 5%. The data and safety monitoring board (DSMB) closed the study to further accrual as a result of the first planned interim analysis for futility which occurred when 174 patients reached day 56.
Detailed response data at days 14, 28, 42, and 56 were tabulated by treatment arm and the proportions experiencing a CR at each time point were compared using the χ2 test. The proportion of patients experiencing flares by day 90 among those who initially achieved a CR were also compared using the χ2 test. Chronic GVHD, incidence of serious, life-threatening, or fatal infection, incidence of Epstein-Barr virus reactivation, transplant-related mortality (TRM), and relapse were described using cumulative incidence and compared between arms using the Gray test, with relapse as the competing event for TRM and death as the competing event for all other outcomes. Disease-free survival (DFS) and OS were described using the Kaplan-Meier estimate and compared between arms using the log-rank test. The median and range of steroid doses at days 28 and 56 were estimated and the distribution of steroid doses was compared between the treatment groups using the Wilcoxon rank sum test. The proportion of patients using topical/nonabsorbable therapy by day 56 was compared using the χ2 test. MDASI symptom and symptom interference scores were described at baseline and day 56 using mean (standard deviation) and compared using the t test. Adjustment for multiple testing was not performed, so secondary end point results are considered exploratory. No preplanned subgroup analyses were conducted. All statistical analyses were performed using SAS, version 9.3.
Ethical considerations
The study was approved by each site’s institutional review board, and written informed consent was obtained from all enrolled patients prior to study-specific procedures in accordance with the Declaration of Helsinki.
Results
Patients
At the second interim analysis (n = 174 patients evaluable), the stopping rule for futility based on the primary end point was triggered and the study was closed after the DSMB reviewed available study data. A total of 236 patients from 36 transplant centers were randomized by the time of study closure (between February 17, 2010 and November 11, 2011). One hundred seventeen patients were assigned to the MMF arm and 119 to the placebo arm. One subject assigned to MMF was determined to be ineligible as he received high-dose prednisone for >72 hours prior enrollment and was removed from all analyses. Randomization was halted on November 17, 2011 when the futility end point was reached. All patients continued to be treated as per protocol. Table 1 lists the demographic characteristics of the patients. Thirty-seven patients (31.9%) on the MMF arm and 41 patients (34.5%) on the placebo arm reported grade 3-4 aGVHD at study entry; 62 patients (53.5%) on the MMF arm and 58 patients (48.7%) on the placebo arm had visceral involvement (Table 2). Primary disease risk was classified according to Center for International Blood and Marrow Transplant Research (CIBMTR) criteria; 162 patients had a classifiable disease risk: the MMF arm had more patients with advanced disease than the placebo arm (30% vs 14.6%, P = .05).
Demographics
| . | MMF . | Placebo . | Total . |
|---|---|---|---|
| Patients | 116 | 119 | 235 |
| AML | 41 (35.3%) | 47 (39.5%) | 88 (37.4%) |
| ALL | 16 (13.8%) | 14 (11.8%) | 30 (12.8%) |
| CML | 3 (2.6%) | 5 (4.2%) | 8 (3.4%) |
| MDS | 20 (17.2%) | 17 (14.3%) | 37 (15.7%) |
| Lymphoma | 17 (14.7%) | 17 (14.3%) | 34 (14.5%) |
| Other | 19 (16.4%) | 19 (16.0%) | 38 (16.2%) |
| Days from BMT to randomization, median (interquartile range) | 36 (24.0-50.0) | 37 (23.0-60.0) | 36 (24.0-55.0) |
| Days from BMT to aGVHD diagnosis, median (interquartile range) | 34 (21-49) | 36 (22-60) | 35 (22-55) |
| Hours from aGVHD diagnosis to randomization, median (interquartile range) | 31 (6-55) | 22 (3-52) | 26 (3-54) |
| Unrelated donor | 66 (56.9%) | 72 (60.5%) | 138 (58.7%) |
| Bone marrow grafts | 23 (19.8%) | 16 (13.4%) | 39 (16.6%) |
| Peripheral blood stem cell grafts | 91 (78.4%) | 102 (85.7%) | 193 (82.1%) |
| Cord blood grafts | 2 (1.7%) | 1(0.8%) | 3 (1.3%) |
| Myeloablative conditioning | 74 (63.8%) | 74 (62.2%) | 148 (63%) |
| Female | 45 (38.8%) | 41 (34.5%) | 86 (36.6%) |
| Male | 71 (61.2%) | 78 (65.5%) | 149 (63.4%) |
| Age, y, median (range, 9.1-76.3) | 54 | 52.9 | 53.8 |
| Patients younger than 18 y of age | 4 (3.4%) | 1 (0.8%) | 5 (2.1%) |
| . | MMF . | Placebo . | Total . |
|---|---|---|---|
| Patients | 116 | 119 | 235 |
| AML | 41 (35.3%) | 47 (39.5%) | 88 (37.4%) |
| ALL | 16 (13.8%) | 14 (11.8%) | 30 (12.8%) |
| CML | 3 (2.6%) | 5 (4.2%) | 8 (3.4%) |
| MDS | 20 (17.2%) | 17 (14.3%) | 37 (15.7%) |
| Lymphoma | 17 (14.7%) | 17 (14.3%) | 34 (14.5%) |
| Other | 19 (16.4%) | 19 (16.0%) | 38 (16.2%) |
| Days from BMT to randomization, median (interquartile range) | 36 (24.0-50.0) | 37 (23.0-60.0) | 36 (24.0-55.0) |
| Days from BMT to aGVHD diagnosis, median (interquartile range) | 34 (21-49) | 36 (22-60) | 35 (22-55) |
| Hours from aGVHD diagnosis to randomization, median (interquartile range) | 31 (6-55) | 22 (3-52) | 26 (3-54) |
| Unrelated donor | 66 (56.9%) | 72 (60.5%) | 138 (58.7%) |
| Bone marrow grafts | 23 (19.8%) | 16 (13.4%) | 39 (16.6%) |
| Peripheral blood stem cell grafts | 91 (78.4%) | 102 (85.7%) | 193 (82.1%) |
| Cord blood grafts | 2 (1.7%) | 1(0.8%) | 3 (1.3%) |
| Myeloablative conditioning | 74 (63.8%) | 74 (62.2%) | 148 (63%) |
| Female | 45 (38.8%) | 41 (34.5%) | 86 (36.6%) |
| Male | 71 (61.2%) | 78 (65.5%) | 149 (63.4%) |
| Age, y, median (range, 9.1-76.3) | 54 | 52.9 | 53.8 |
| Patients younger than 18 y of age | 4 (3.4%) | 1 (0.8%) | 5 (2.1%) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMT, bone marrow transplantation; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome.
GVHD characteristics for the group
| . | N (%) . | ||
|---|---|---|---|
| MMF . | Placebo . | Total . | |
| Total enrolled | 116 | 119 | 235 |
| Grade of de novo aGVHD at diagnosis | |||
| Grade I | 11 (9.5) | 16 (13.4) | 27 (11.5) |
| Grade II | 68 (58.6) | 62 (52.1) | 130 (55.3) |
| Grade III | 30 (25.9) | 34 (28.6) | 64 (27.2) |
| Grade IV | 7 (6) | 7 (5.9) | 14 (6) |
| Cutaneous involvement at onset | |||
| No rash | 31 (26.7) | 36 (30.3) | 67 (28.5) |
| Maculopapular rash, <25% of body surface | 11 (9.5) | 14 (11.8) | 25 (10.6) |
| Maculopapular rash, 25%-50% of body surface | 27 (23.3) | 21 (17.6) | 48 (20.4) |
| Generalized erythroderma | 46 (39.7) | 44 (37) | 90 (38.3) |
| Generalized erythroderma with bullae formation and desquamation | 1 (0.9) | 4 (3.4) | 5 (2.1) |
| Upper GI abnormalities at diagnosis | |||
| No protracted nausea and vomiting | 85 (73.3) | 82 (68.9) | 167 (71.1) |
| Persistent nausea, vomiting, or anorexia | 31 (26.7) | 37 (31.1) | 68 (28.9) |
| Lower GI abnormalities at diagnosis | |||
| No diarrhea | 57 (49.1) | 66 (55.5) | 123 (52.3) |
| Diarrhea ≤500 mL/d or <280 mL/m2 | 21 (18.1) | 19 (16) | 40 (17) |
| Diarrhea >500 but ≤1000 mL/d or 280-555 mL/m2 | 21 (18.1) | 14 (11.8) | 35 (14.9) |
| Diarrhea >1000 but ≤1500 mL/d or 556-833 mL/m2 | 8 (6.9) | 9 (7.6) | 17 (7.2) |
| Diarrhea >1500 mL/d or >833 mL/m2 | 7 (6) | 11 (9.2) | 18 (7.7) |
| Severe abdominal pain with or without ileus, or stool with frank blood or melena | 2 (1.7) | 0 (0) | 2 (0.9) |
| Liver abnormalities at diagnosis, bilirubin, mg/dL | |||
| <2 | 106 (91.4) | 110 (92.4) | 216 (91.9) |
| 2-3 | 1 (0.9) | 5 (4.2) | 6 (2.6) |
| 3.1-6 | 6 (5.2) | 4 (3.4) | 10 (4.3) |
| 6.1-15 | 2 (1.7) | 0 (0) | 2 (0.9) |
| >15 | 1 (0.9) | 0 (0) | 1 (0.4) |
| . | N (%) . | ||
|---|---|---|---|
| MMF . | Placebo . | Total . | |
| Total enrolled | 116 | 119 | 235 |
| Grade of de novo aGVHD at diagnosis | |||
| Grade I | 11 (9.5) | 16 (13.4) | 27 (11.5) |
| Grade II | 68 (58.6) | 62 (52.1) | 130 (55.3) |
| Grade III | 30 (25.9) | 34 (28.6) | 64 (27.2) |
| Grade IV | 7 (6) | 7 (5.9) | 14 (6) |
| Cutaneous involvement at onset | |||
| No rash | 31 (26.7) | 36 (30.3) | 67 (28.5) |
| Maculopapular rash, <25% of body surface | 11 (9.5) | 14 (11.8) | 25 (10.6) |
| Maculopapular rash, 25%-50% of body surface | 27 (23.3) | 21 (17.6) | 48 (20.4) |
| Generalized erythroderma | 46 (39.7) | 44 (37) | 90 (38.3) |
| Generalized erythroderma with bullae formation and desquamation | 1 (0.9) | 4 (3.4) | 5 (2.1) |
| Upper GI abnormalities at diagnosis | |||
| No protracted nausea and vomiting | 85 (73.3) | 82 (68.9) | 167 (71.1) |
| Persistent nausea, vomiting, or anorexia | 31 (26.7) | 37 (31.1) | 68 (28.9) |
| Lower GI abnormalities at diagnosis | |||
| No diarrhea | 57 (49.1) | 66 (55.5) | 123 (52.3) |
| Diarrhea ≤500 mL/d or <280 mL/m2 | 21 (18.1) | 19 (16) | 40 (17) |
| Diarrhea >500 but ≤1000 mL/d or 280-555 mL/m2 | 21 (18.1) | 14 (11.8) | 35 (14.9) |
| Diarrhea >1000 but ≤1500 mL/d or 556-833 mL/m2 | 8 (6.9) | 9 (7.6) | 17 (7.2) |
| Diarrhea >1500 mL/d or >833 mL/m2 | 7 (6) | 11 (9.2) | 18 (7.7) |
| Severe abdominal pain with or without ileus, or stool with frank blood or melena | 2 (1.7) | 0 (0) | 2 (0.9) |
| Liver abnormalities at diagnosis, bilirubin, mg/dL | |||
| <2 | 106 (91.4) | 110 (92.4) | 216 (91.9) |
| 2-3 | 1 (0.9) | 5 (4.2) | 6 (2.6) |
| 3.1-6 | 6 (5.2) | 4 (3.4) | 10 (4.3) |
| 6.1-15 | 2 (1.7) | 0 (0) | 2 (0.9) |
| >15 | 1 (0.9) | 0 (0) | 1 (0.4) |
GVHD response
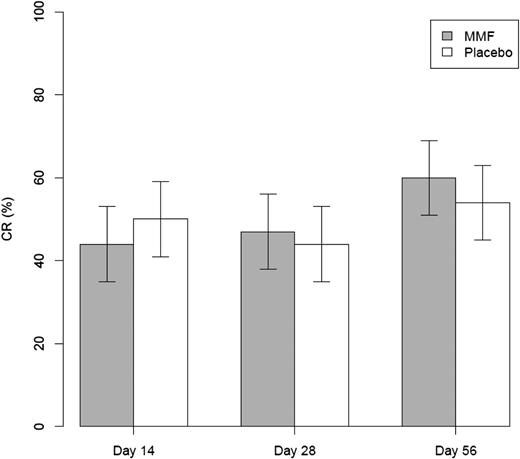
Among the 235 eligible patients at the time of data cutoff, 69 (59.5%; 95% confidence interval [CI], 50.0%-68.4%) on the MMF arm and 60 (50.4%; 95% CI, 41.4%-59.4%) in the placebo arm were alive and free of GVHD at day 56 after randomization (P = .16) (Table 3). There were no differences in day 56 GVHD-free survival in patients with grades 3-4 (MMF, 54.1%; placebo, 51.2%; P = .8) nor in patients with liver/gut involvement (MMF, 51.6%; placebo, 60.3%, P = .34) at onset. CR rate at day 56 was similar: MMF 60.3% vs placebo 53.8% (P = .31). CR rates at days 14 and 28 were similar in the 2 arms, 44% vs 49.6%, and 46.6% vs 44.5%, respectively, for MMF vs placebo (P = .39 and .76, respectively) (Figure 2). Of the 174 patients who had a CR at 1 time point, 8 (9%) on the MMF arm and 16 (18.8%) on the placebo arm had a flare of GVHD prior to day 90 (P = .06). No statistically significant differences were seen in the incidence of cGVHD. The cumulative incidence of cGVHD at 12 months was 41.5% (95% CI, 32.3-50.7) and 43.3% (95% CI, 34.2-52.4) for the MMF and placebo arms, respectively (P = .69). Both treatment groups were able to taper the prednisone or equivalent corticosteroid dosing between days 28 and 56. The corticosteroid dose in the MMF group decreased from a median of 0.60 mg/kg to 0.17 mg/kg whereas the placebo group decreased from a median of 0.63 mg/kg to 0.20 mg/kg. The corticosteroid doses at day 28 and day 56 were not different between treatment groups (P = .70 and .59, respectively). Seventy-seven subjects (66.4%) on the MMF arm and 81 subjects (68.1%) on the placebo arm reported the use of topical corticosteroid therapy (P = .712).
Success is defined as alive without aGVHD or cGVHD at day 56 after randomization
| . | Treatment arm, n (%) . | |
|---|---|---|
| Day 56 GVHD-free survival status . | MMF, N = 116 . | Placebo, N = 119 . |
| Success* | 69 (59.5) | 60 (50.4) |
| Failure | 47 (40.5) | 59 (47.8) |
| Died prior to day 56 | 11 (9) | 13 (10.9) |
| Alive with aGVHD | 12 (10.3) | 9 (7.5) |
| Alive with cGVHD | 1 (<1) | 4 (3.3) |
| Alive with systemic drug added or escalation of corticosteroids | 21 (18.1) | 29 (24.3) |
| Lost to follow-up or withdrew consent | 2 (1.7) | 4 (3.3) |
| Total evaluable | 116 | 119 |
| . | Treatment arm, n (%) . | |
|---|---|---|
| Day 56 GVHD-free survival status . | MMF, N = 116 . | Placebo, N = 119 . |
| Success* | 69 (59.5) | 60 (50.4) |
| Failure | 47 (40.5) | 59 (47.8) |
| Died prior to day 56 | 11 (9) | 13 (10.9) |
| Alive with aGVHD | 12 (10.3) | 9 (7.5) |
| Alive with cGVHD | 1 (<1) | 4 (3.3) |
| Alive with systemic drug added or escalation of corticosteroids | 21 (18.1) | 29 (24.3) |
| Lost to follow-up or withdrew consent | 2 (1.7) | 4 (3.3) |
| Total evaluable | 116 | 119 |
P = .16.
Toxicities
Three serious unexpected AEs were recorded in each group (MMF: cerebellar ataxia, orthostasis, and dehydration; placebo: small-bowel fistula, septic shock, and posterior reversible encephalopathy syndrome). Through day 56, 79.8% of patients in the MMF arm reported a grade 3-5 toxicity compared with 76.5% in the placebo arm. The only toxicity that was significantly different between both groups was a higher incidence of leukopenia on the MMF arm (MMF, 52.7%; placebo, 34.8%; P = .01). Eighty-one patients (69.8%) treated with MMF experienced at least 1 infection compared with 77 patients (64.7%) on the placebo arm (P = .4). The cumulative incidence of a severe/life-threatening/fatal infection at 12 months was 44.5% (95% CI, 35.3- 53.6) for MMF and was 42.9% (95% CI, 33.9-52.0) for placebo (P = .83). Forty-four percent of viral infections on the MMF arm and 39% of viral infections on the placebo arm were due to cytomegalovirus (P = .53). Six patients on the MMF arm and 4 patients on the placebo arm developed Epstein-Barr virus reactivation after randomization (P = .50). Overall, 66.4% of patients on the MMF arm and 62.2% of patients on the placebo arm had a least 1 readmission. There was no difference in compliance with taking the study drug, 94 patients in each group (81% MMF, 79% placebo, P = .69) were compliant with their medication for at least 2 weeks. Between day 0 and day 28, 58 of MMF subjects (51.3%) and 58 of placebo subjects (50.0%) reported a dose being reduced or withheld. Between day 28 and day 56, 26 of 61 MMF subjects (42.6%) and 29 of 63 placebo subjects (43.0%) reported a dose being reduced or withheld.
DFS and OS
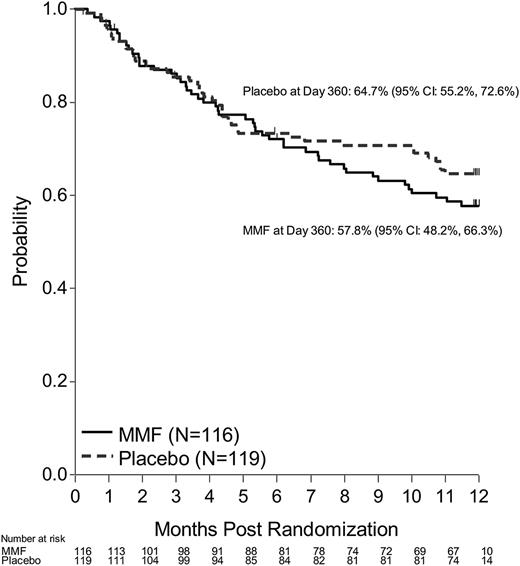
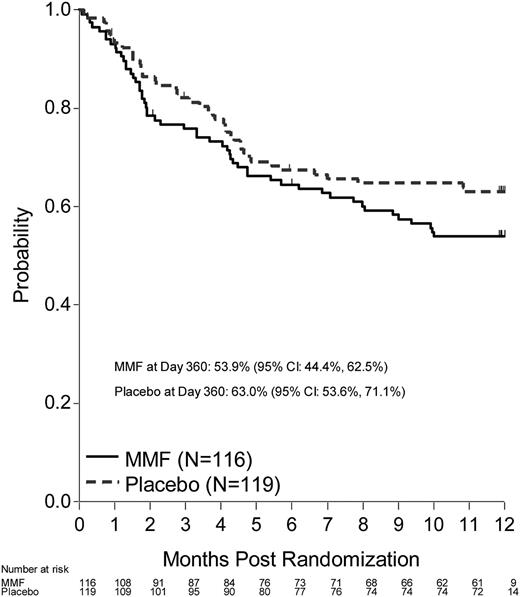
DFS (including death or progression/relapse of malignancy) probability at 12 months after randomization was 53.9% (95% CI, 44.4-62.5) for the MMF arm and 63% (95% CI, 53.6-71.1) for the placebo arm (P = .17). OS probability at 6 months after randomization was 72% (95% CI, 62.8-79.3) for the MMF arm and 73.4% (95% CI, 64.4-80.5) for the placebo arm (Figures 3-4). Corresponding OS probabilities at 12 months after randomization were 57.8% (95% CI, 48.2-66.3) and 64.7% (95% CI, 55.2-72.6) (P = .34). Relapse (47.9%) was the most common cause of death among patients treated with MMF followed by aGVHD (35.4%). aGVHD (43.9%) was the most common cause of death reported among patients who received placebo followed by relapse (39%). At 12 months, the cumulative incidence of relapse for the MMF arm was 24.3% (95% CI, 16.4-32.2) and 15.5% (95% CI, 8.9-22.1) on the placebo arm (P = .08). TRM at 12 months (MMF: 21.8%, 95% CI, 14.2-29.4 vs placebo 21.5%, 95% CI, 14-29) did not differ between groups (P = 1.00).
Quality of life
The results of the MDASI were scored at day 0 and day 56 (MMF 103 patients, placebo 108). There was no difference between the treatment arms in either total symptom score severity or symptom interference score at day 0, day 56, or change between day 0 and day 56. On average, both arms reported a lower score at day 56.
Discussion
This multicenter randomized double-blinded, phase 3 trial demonstrated that MMF added to corticosteroids did not provide a benefit in GVHD-free survival when compared with corticosteroids alone. In our balanced cohorts, we observed no differences in day 56 GVHD-free survival, cGVHD, OS, or DFS at 6 or 12 months after randomization. These results indicate that MMF should not be combined with corticosteroids as primary therapy for aGVHD, at least in the broad range of GVHD patients as reflected in this trial.
Previous randomized phase 3 studies testing the combination of additional immunosuppressive agents with corticosteroids as initial therapy of aGVHD have similarly failed to demonstrate any response or survival advantage.4,6,8,9 Anti–interleukin 2 receptor (IL2R) monoclonal antibodies, ATG, and infliximab have all failed to show any benefit when combined to steroids and compared with steroids alone in randomized phase 3 studies.4,6,8,9 Taken together with these studies, our results raise the question of whether escalation of broad immune suppression early in the treatment of acute GVHD will ultimately achieve better GVHD control and survival. Although this approach may still be valid, it is possible that future endeavors may have to focus on agents that have more specific immune-suppressive activity or aimed at other novel targets that could improve GVHD response without increasing the risk of complications.13
A potential limitation of our study is that it used broad eligibility criteria for GVHD severity, including all patients deemed to require systemic therapy of GVHD. Although randomization was stratified by GVHD grade to assure balance of GVHD severity in the 2 study arms, we acknowledge that the inclusion of “low”-risk patients (eg, grade I-II skin, or grade II upper GI only), who might do very well with corticosteroids alone, could have impaired our ability to discern a benefit for the addition of MMF. However, we also did not observe any benefit for the addition of MMF among patients with grade III-IV or visceral GVHD. Despite these results, it is likely that there exist subsets of aGVHD patients who are destined to do poorly with corticosteroids alone, and who could benefit from more aggressive therapy. Recent data suggest that blood biomarkers taken early after HSCT can predict the development of aGVHD as well as response to corticosteroid therapy.14,15 Therefore, it is conceivable that these biomarkers could be used as tools for identifying patients with “high-risk” aGVHD for clinical trials of combinational initial therapy. It also has to be remembered that this study enrolled very few children and patients undergoing cord transplants.
These considerations notwithstanding, the results of this trial provide another reminder of the importance of performing phase 3 randomized placebo-controlled studies to validate promising phase 2 data. Until definitive data indicates otherwise, current standard treatment of de novo aGVHD after HSCT should remain corticosteroids alone, as the addition of MMF, does not improve treatment outcomes or survival.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The National Heart, Lung, and Blood Institute (NHLBI) statisticians had access to the analyses provided to the DSMB but did not participate in the DSMB’s decisions. The NHLBI reviewed this report and offered input.
This work was supported by grant #U10HL069294 from the NHLBI and the National Cancer Institute at the National Institutes of Health.
Authorship
Contribution: J.B.-M., V.T.H., and B.R.L. designed the study protocol and wrote the paper; J.H.A., M.M.H., P.J.M., and D.J.W. were senior advisors in the design and conduct of the study; J.B.-M., B.R.L., V.T.H., A.M.A., K.B., S.C.G., J.E.L., M.L.M., R.N., and M.C.P. formed the end point review committee; J.B.-M., V.T.H., A.M.A., J.H.A., S.C.G., E.O.H., M.M.H., S.J.L., J.E.L., M.L.M., P.J.M., R.N., M.C.P., D.J.W., and P.W. enrolled and cared for patients and analyzed data; B.R.L., S.L.C., and A.M.M. provided statistical analysis; all authors reviewed and provided insightful comments to better the manuscript; and B.R.L. and A.M.M. drew the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Javier Bolaños-Meade, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, CRB-I 2M87, 1650 Orleans St, Baltimore, MD 21231; e-mail fbolano2@jhmi.edu; and Vincent T. Ho, Dana-Farber Cancer Institute, 450 Brookline Ave, D2030, Boston, MA 02215; e-mail: vincent_ho@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal